DEFINING THE ATOM SIZING UP THE ATOM Dalton









- Slides: 19

DEFINING THE ATOM

SIZING UP THE ATOM • Dalton believed if you took an element and kept breaking it apart over and over, eventually you would get to a piece of the element you couldn’t break apart anymore. • This smallest part of an element is the atom • Atoms are extremely small • Most atomic radii are between 5*10 -11 m and 2*10 -10 m. • A copper coin the size of a penny has approximately 2. 4*1022 atoms in it. • Scanning Tunneling Electron Microscopes allow us to see things on this scale • We even have the ability to move individual atoms around to create new and important new materials

STRUCTURE OF THE NUCLEAR ATOM By Presenter. Media. com

SUBATOMIC PARTICLES • Dalton was mostly right. • One exception: atoms are divisible • There are 3 subatomic particles: 1. Electrons 2. Protons 3. Neutrons

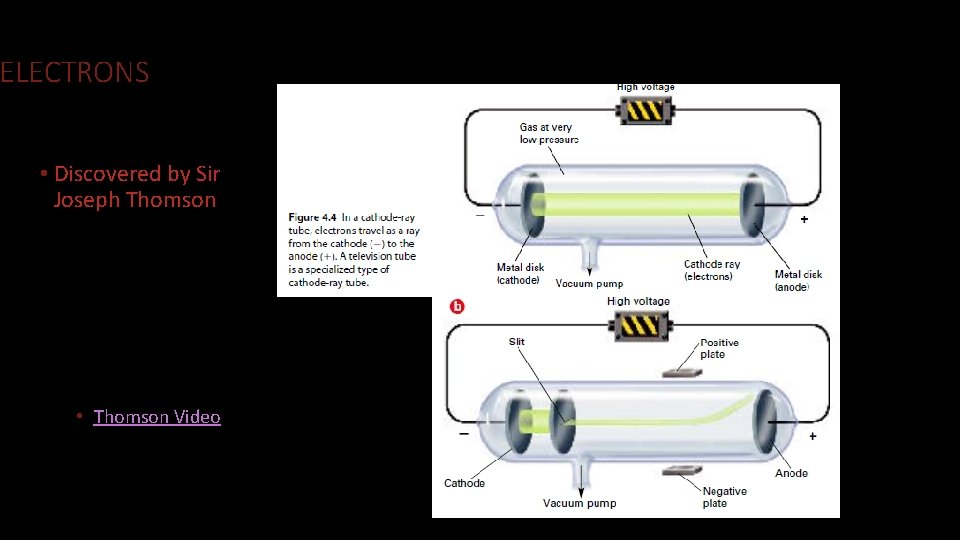
ELECTRONS • Discovered by Sir Joseph Thomson • Thomson Video

ELECTRONS • Thomson realized a few things • Something was attracted to the positively charged plate • What did this mean was in the atom? • He found that the charge to mass ratio was constant. • And that it did not matter what gas was in the electrode. • What does this tell us about all atoms?

ATOMS AND THEIR CHARGES • Atoms have no net charge • Electrically neutral • Electric charges are carried by particles of matter • Electric charges exist in whole number ratios • Not fractional charges • +1, +2, 0, -1, -2 etc. • When equal numbers of positively charged particles and negatively charged particles combine, an electrically neutral particle is formed.

PROTONS • Think about this, if an atom is electrically neutral, what happens when you take away an electron ( a-1 charge)? • What charge is left? • A Hydrogen atom has one proton and one electron • What must the charge of a proton be, if it has to counteract the negative charge of an electron?

DISTINGUISHING AMONG ATOMS

ESSENTIAL QUESTIONS

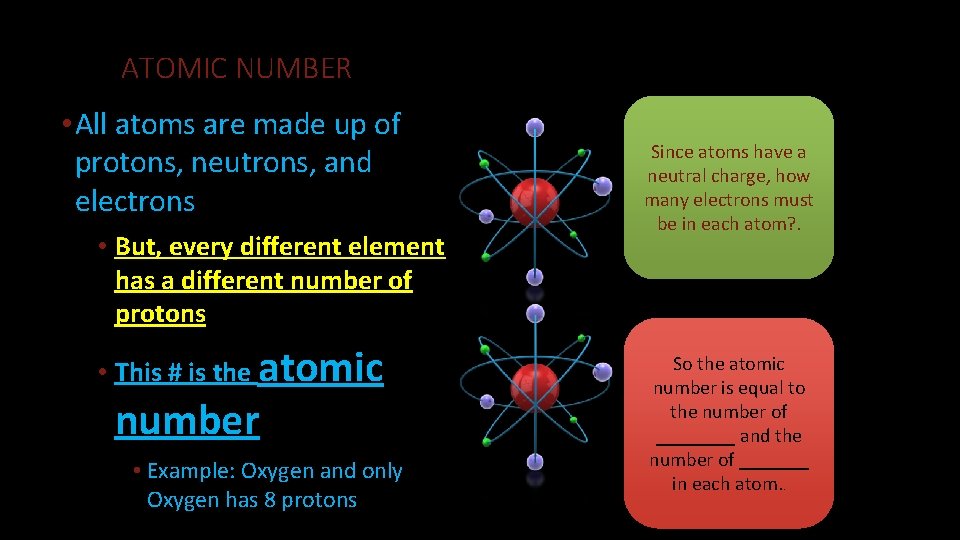
ATOMIC NUMBER • All atoms are made up of protons, neutrons, and electrons • But, every different element has a different number of protons • This # is the atomic number • Example: Oxygen and only Oxygen has 8 protons Since atoms have a neutral charge, how many electrons must be in each atom? . So the atomic number is equal to the number of ____ and the number of _______ in each atom. .

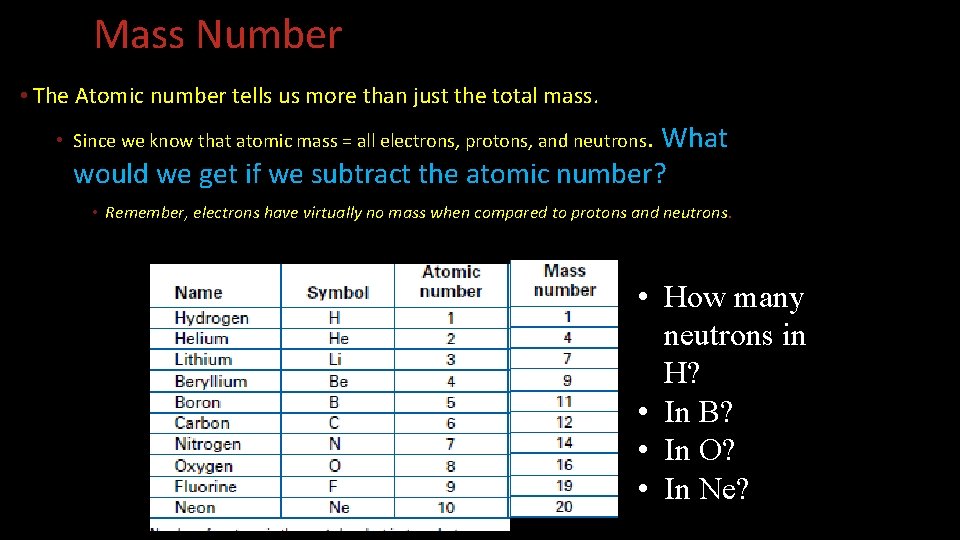
MASS NUMBER • We know the atomic # tells us the number of H+ (protons) in an atom and the number of e (electrons). • Mass Number is more than that. It is how much matter that is in the atom. • Protons, electrons, and Neutrons. • Since Protons and Neutrons are the majority of the mass, the number is close to the number of protons and neutrons added together. (we will talk about the difference later)

Mass Number • The Atomic number tells us more than just the total mass. • Since we know that atomic mass = all electrons, protons, and neutrons. What would we get if we subtract the atomic number? • Remember, electrons have virtually no mass when compared to protons and neutrons. • How many neutrons in H? • In B? • In O? • In Ne?

Mass Number • If Uranium has an Mass Number of 235 and 92 e- , what is the atomic #? • Sometimes chemists write atomic mass, atomic number, and chemical symbol in a shorthand fashion. • For example: • Which is the big #, • Mass#?

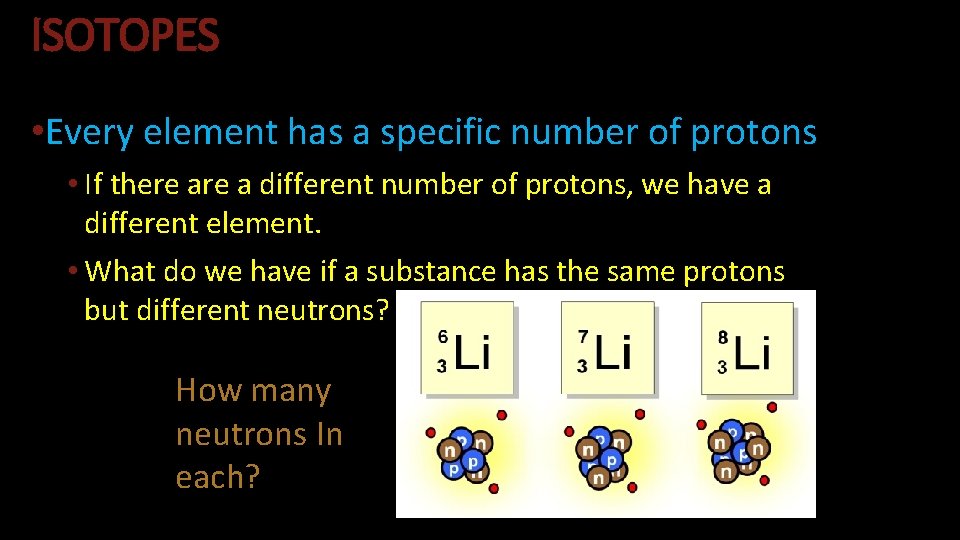
ISOTOPES • Every element has a specific number of protons • If there a different number of protons, we have a different element. • What do we have if a substance has the same protons but different neutrons? How many neutrons In each?

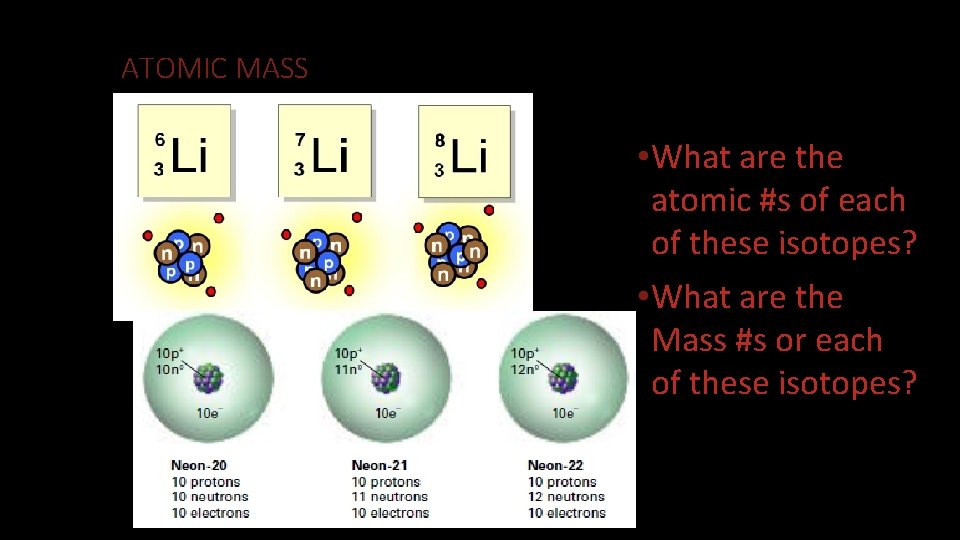
ATOMIC MASS • What are the atomic #s of each of these isotopes? • What are the Mass #s or each of these isotopes?

ATOMIC MASS • Subatomic particles are extremely small. • The mass of a proton is about 1. 67*10 -24 g • The mass of a electron is about 9. 11*10 -28 g • These numbers are really hard to use all the time, so scientists decided to use a standard reference. • Carbon-12 (the most common isotope) has 6 protons and 6 neutrons. These scientists decided to make this element equal 12 atomic mass units (AMU)s • 1 AMU = 1/12 of the mass of a Carbon-12 atom

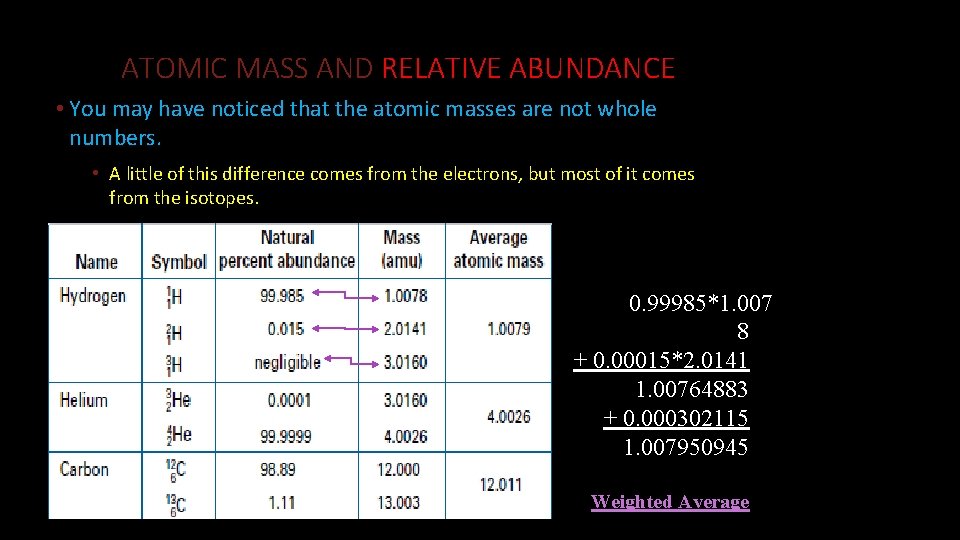
ATOMIC MASS AND RELATIVE ABUNDANCE • You may have noticed that the atomic masses are not whole numbers. • A little of this difference comes from the electrons, but most of it comes from the isotopes. 0. 99985*1. 007 8 + 0. 00015*2. 0141 1. 00764883 + 0. 000302115 1. 007950945 Weighted Average

ESSENTIAL QUESTIONS