Defining Matter Day 2 Summer School Warmup in
























- Slides: 31

Defining Matter Day 2, Summer School

Warm-up (in your spiral notebook) Determine the number of sig figs in the following: ◦. 010 ◦ 50700 Round the following measurements to two significant figures: ◦. 0719 ◦ 5406

Warm-up part II Write the following numbers using scientific notation with the correct number of sig figs: ◦. 00065020 ◦ 2780000 Expand the following numbers with the correct number of significant figures: ◦ 2. 34 x 105 ◦ 3. 1010 x 10 -2

Warm-up part III Make the following calculations rounding your answer to the correct number of sig figs: ◦ ◦ 2. 350 x. 00123 1. 23 x 105 x 1. 0 x 107 23. 45 + 17. 5 + 13 1. 23 x 105 + 1. 0 x 107

Day 2 - June 25, 2013 Review HW Sig Fig Quiz Intro to Lab Notebook All That Glitters Lab Defining Matter Test A New Language Activity & Notes

All That Glitters In the year 1 BC, King Hiero commissioned the creation of a golden crown. However, he didn’t believe that the goldsmith used all gold. He asked Archimedes to determine if the crown was solid gold.

All That Glitters Do you think Archimedes can determine if the crown is solid gold by putting it under water? Why or why not? What happens to the level of the water if you submerge a crown in the water?

All That Glitters Mass ◦ is the amount of stuff or substance. We measure mass by measuring weight on a scale or balance. ◦ Careful: Mass = Weight Volume ◦ refers to the amount of space occupied by a substance.

All That Glitters Density ◦ how much “stuff” there is in a certain space. ◦ D = M/V Intrinsic Property ◦ a property of the substance that doesn’t depend on size and shape.

Density D ( ) = Mass/Volume measured in: grams (g)/ml It gives you an idea of how tightly packed the matter is in an object Every substance on earth has its own unique density

Density A gold miner has just found a nugget of pure gold. He measures its dimensions and then calculates its volume to be 0. 125 L. Knowing that the density of gold is 19. 3 g / ml, calculate the mass of the miner’s nugget.

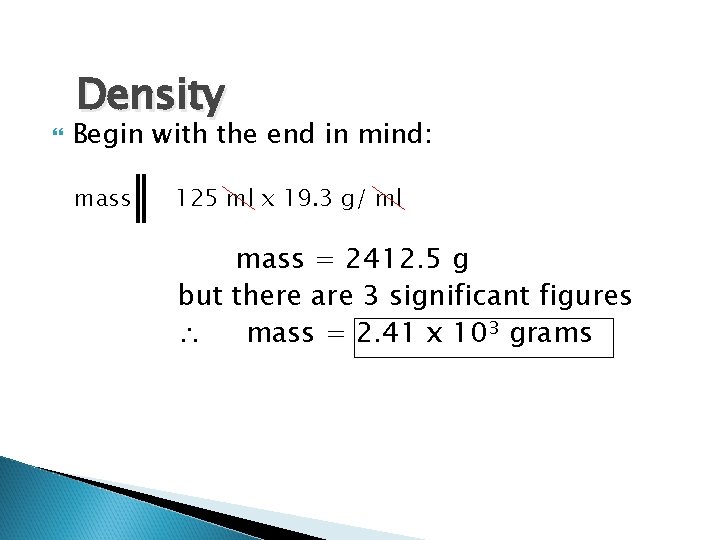
Density What is your unknown? (What is your end in mind? ) ◦ Mass in grams List your Givens: ◦ Volume = 0. 125 Liters ◦ Density = 19. 3 g / ml

Density Begin with the end in mind: mass 125 ml x 19. 3 g/ ml mass = 2412. 5 g but there are 3 significant figures mass = 2. 41 x 103 grams

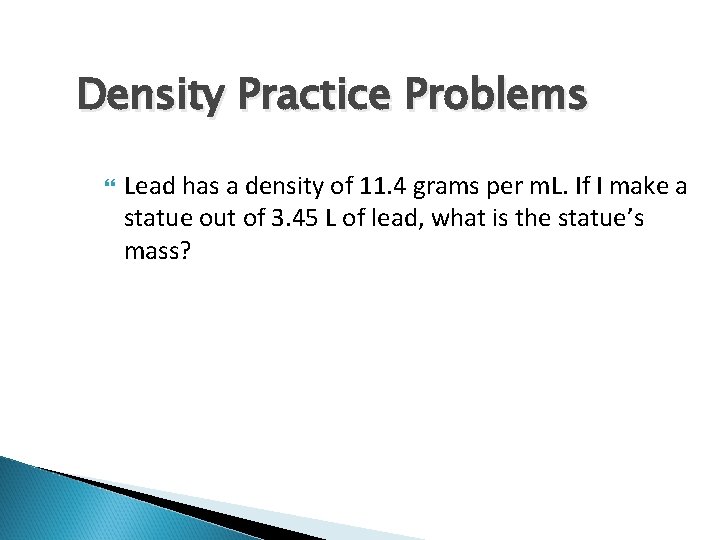
Density Practice Problems Lead has a density of 11. 4 grams per m. L. If I make a statue out of 3. 45 L of lead, what is the statue’s mass?

Density Practice Problem A gold miner tries to sell some gold that he found in a nearby river. The person who is thinking about purchasing the gold measures the mass and volume of one nugget. The mass is 0. 319 kg and the volume is 0. 065 liters. Is this nugget really made out of gold? (Remember that the density of gold is 19. 3 grams per m. L)

Catalyst There are two bottles on a shelf in a chemistry lab. Both contain a shiny metal substance that resembles gold. Bottle A is labeled Au (s). Bottle B is labeled Fe. S 2 (s). ◦ Do you think both bottles contain gold? Why or why not? ◦ What do you think the symbols on the bottles mean?

What is an element? Elements are substances containing only one type of atom. Elements are the simplest forms of matter that can exist under normal laboratory conditions. ◦ Cannot be separated into simpler substances ◦ Building blocks for all substances

What are atoms? Fundamental unit of which elements are made Not all atoms are the same There are over 100 different types of atoms in the universe. They are called………

A New Language What do the chemical symbols tell us about the substance inside the bottle? Draw Data Table that is on page 18 of Alchemy Collect Data Complete Questions 1 – 8 only after collecting data & returning to your seats.

Making Sense of A New Language What symbols are used to label something containing sulfate? Nitrate? Hydroxide? What symbols represent copper sulfate? Is copper sulfate an element or a compound? How do you know? What do you think the subscript numbers mean?

What is a substance? A particular kind of matter that has a uniform and definite composition. Examples: sugar, water, elements, compounds

What are compounds? Compounds is a substance that consists of two or more elements chemically combined together. Can be separated into simpler substances only by chemical reactions.

What are compounds? A compound always contains atoms of different elements A compound, although it contains more than one type of atom, always has the same composition (same combination of atoms)

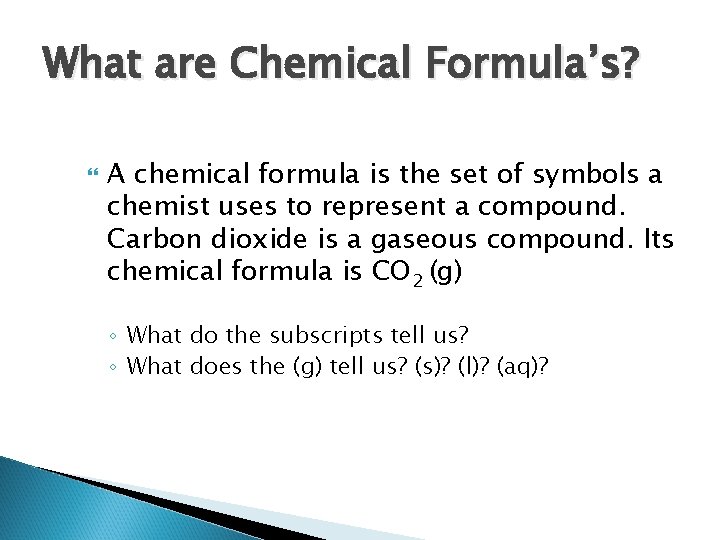
What are Chemical Formula’s? A chemical formula is the set of symbols a chemist uses to represent a compound. Carbon dioxide is a gaseous compound. Its chemical formula is CO 2 (g) ◦ What do the subscripts tell us? ◦ What does the (g) tell us? (s)? (l)? (aq)?

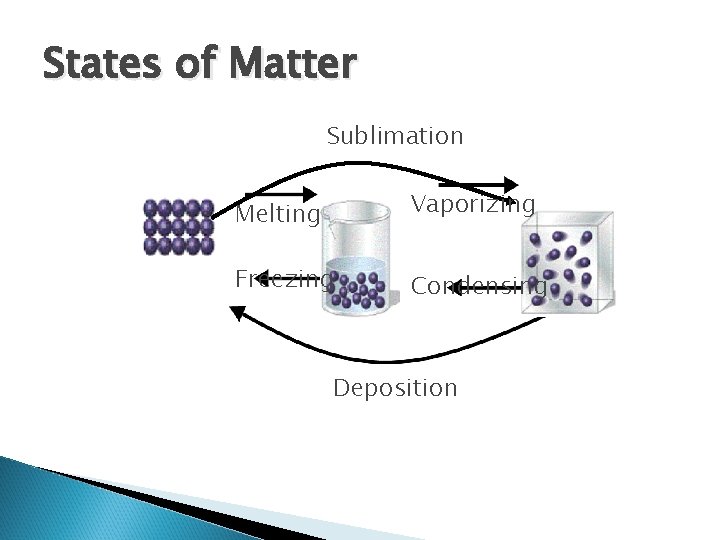
States of Matter Sublimation Melting Vaporizing Freezing Condensing Deposition

What does aqueous mean? A substance is aqueous if it is dissolved in water. ◦ The substance that is dissolved is the solute. ◦ The water is referred to as the solvent. It does the dissolving.

Property A quality or trait that identifies a substance is called a property.

What are physical properties? Characteristic of a substance that can change without the substance’s becoming a different substance

What are chemical properties? Characteristic that describes the ability of a substance to change to a different substance. Not easily reversed. You have NEW substances after a chemical reaction.

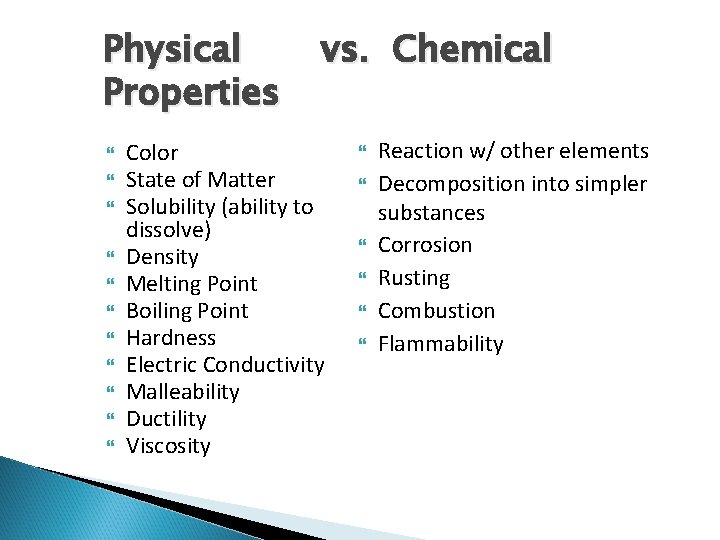
Physical Properties vs. Chemical Color State of Matter Solubility (ability to dissolve) Density Melting Point Boiling Point Hardness Electric Conductivity Malleability Ductility Viscosity Reaction w/ other elements Decomposition into simpler substances Corrosion Rusting Combustion Flammability

Day 2 - Homework HW ◦ ◦ ◦ Complete “All That Glitters” Lab Complete Measurement & Density HW Read Modern Chem pages 6 - 14 Begin memorizing elements Compete Basic Building Materials WS