Define electrolysis The breakdown of an ionic compound
Define electrolysis

The breakdown of an ionic compound, molten or in aqueous solution, by the passage of electricity
What is an ion?

An atom that has lost or gained electrons

What material can be used to make an inert electrode?

graphite

Which type of ion is attracted to the cathode and which is attracted to the anode?
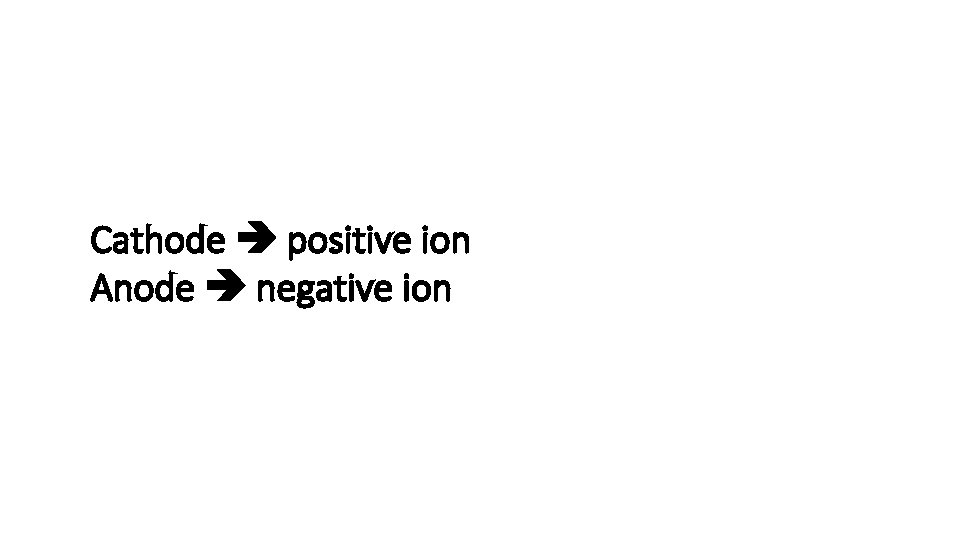
Cathode positive ion Anode negative ion

• Aqueous or molten ionic compounds as the ions are free to move and carry a charge • Solid ionic compounds or non ionic compounds such as sugar or methane will not conduct electricity as there are no free ions to move and carry a charge

State the electrode products from the electrolysis of: • • • molten lead(II) bromide concentrated hydrochloric acid concentrated aqueous sodium chloride Dilute aqueous sodium chloride (brine) dilute sulfuric acid
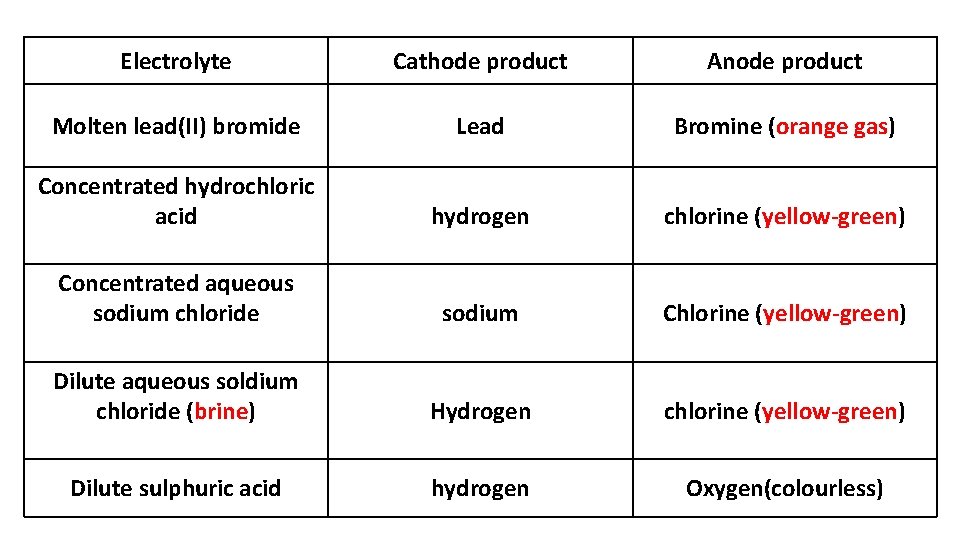
Electrolyte Cathode product Anode product Molten lead(II) bromide Lead Bromine (orange gas) Concentrated hydrochloric acid hydrogen chlorine (yellow-green) Concentrated aqueous sodium chloride sodium Chlorine (yellow-green) Dilute aqueous soldium chloride (brine) Hydrogen chlorine (yellow-green) Dilute sulphuric acid hydrogen Oxygen(colourless)

During electrolysis what is formed at the: • negative electrode (cathode) • positive electrode (anode)
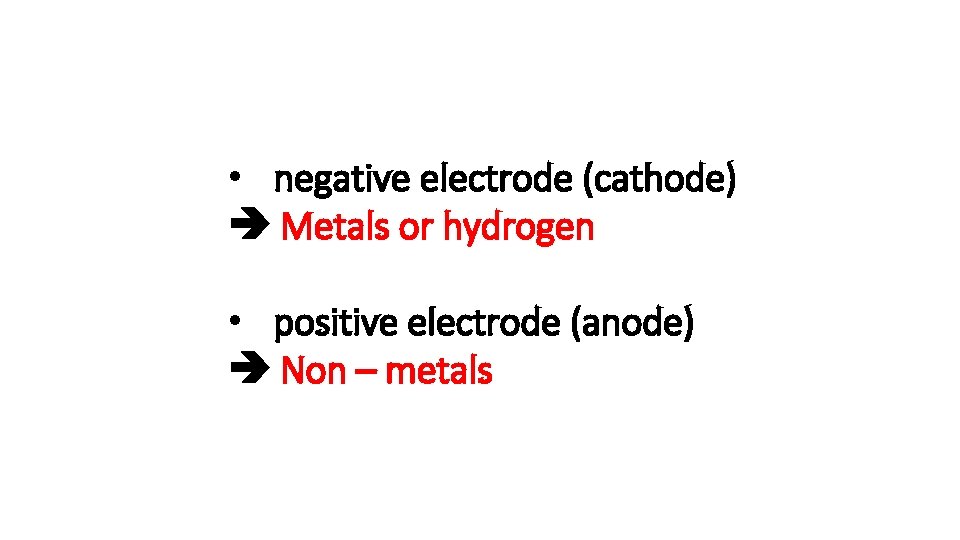
• negative electrode (cathode) Metals or hydrogen • positive electrode (anode) Non – metals

Predict the products of electrolysis of the following molten binary compound : • Molten lead (II) bromide • Molten sodium iodide • Molten zinc chloride
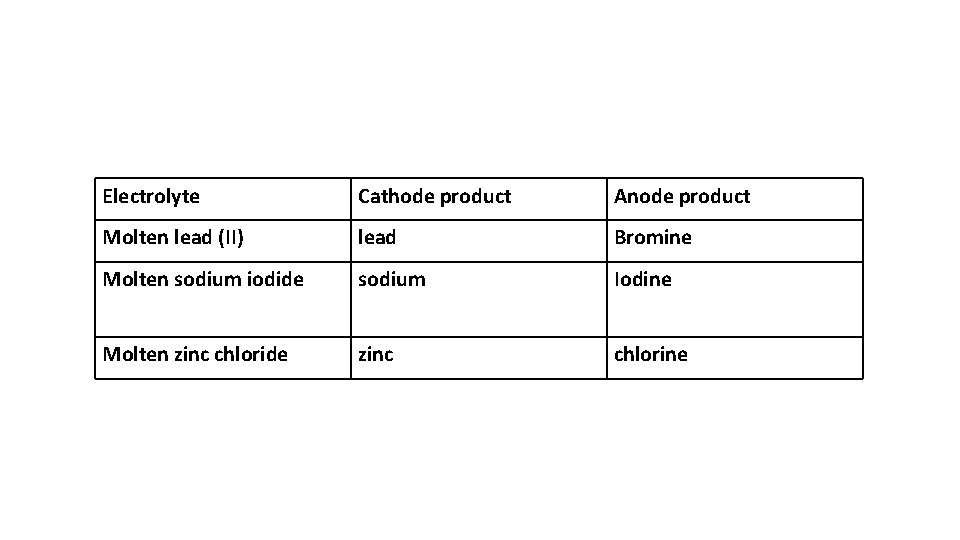
Electrolyte Cathode product Anode product Molten lead (II) lead Bromine Molten sodium iodide sodium Iodine Molten zinc chloride zinc chlorine

What will happen to a light bulb in a circuit with lead bromide when the lead bromide is heated to melting point?

When the lead bromide melts it will be able to conduct electricity and the bulb will light

State 2 safety precautions to take when carrying out the electrolysis of lead bromide

• Carry out in a fume cupboard • Where googles

• What is meant by the terms • oxidation • reduction

• Oxidation loss of electrons (gain of oxygen) • Reduction gain of electrons (removal of oxygen)

Describe the reactions at the electrodes during electrolysis at the cathode

• Positive ions in the electrolyte move to the negative cathode • The positive ions accept electrons from the cathode • This is a reduction reaction because electrons are gained • Metals or hydrogen are formed Zn 2+ + 2 e- Zn 2 H+ + 2 e- H 2

Describe the reactions at the electrodes during electrolysis at the anode

• Negative ions in the electrolyte move to the anode • If the anode is inert the negative ions lose electrons to the anode • Non-metals or oxygen are formed • This is an oxidation reaction because electrons are lost 2 Br- Br 2 +2 e 4 OH- O 2 +2 H 2 O +4 e • If the anode is a reactive electrode the metal atoms of the anode loose electrons and form positive ions • The positive ions go into solution and the anode becomes smaller Cu 2+ +2 e-

Describe how you could predict products of electrolysis of a specified halide in dilute or concentrated aqueous solution

For anions , a concentrated ion tends to get discharged rather than a less concentrated ion Example: • When a concentrated aqueous solution of sodium chloride is electrolysed Cl- ions are discharged and not OH- ions • But if the sodium chloride solution is dilute the OH- ions is discharged. So oxygen is formed and only a little chlorine

Describe the purification of copper
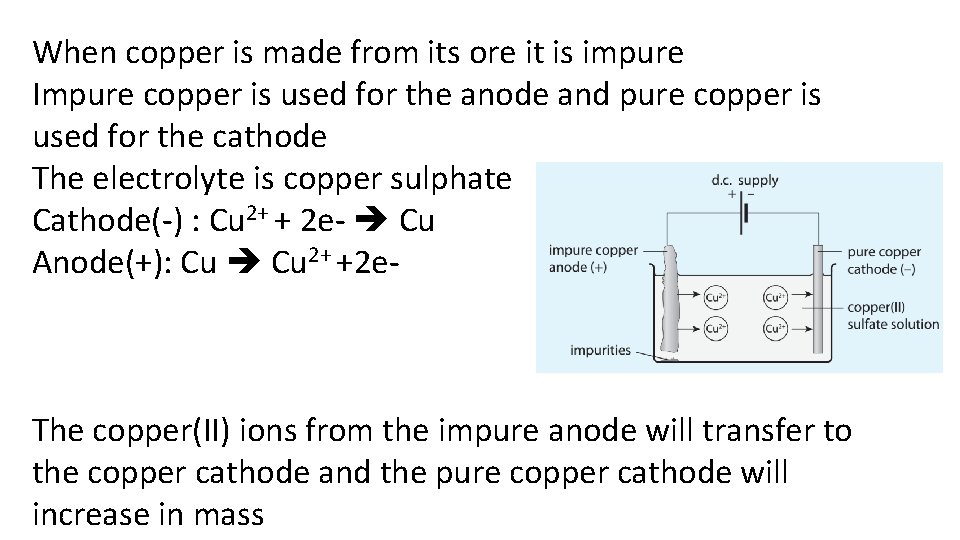
When copper is made from its ore it is impure Impure copper is used for the anode and pure copper is used for the cathode The electrolyte is copper sulphate Cathode(-) : Cu 2+ + 2 e- Cu Anode(+): Cu 2+ +2 e- The copper(II) ions from the impure anode will transfer to the copper cathode and the pure copper cathode will increase in mass

During the purification of copper by electrolysis the copper anode gradually becomes smaller explain why

Copper forms Cu 2+ ions which go into solution

Why is copper used for wires in electrical circuits

Good conductor of electricity

Describe electrolysis of copper sulfate solution with inert graphite electrode

If carbon electrodes are used in the electrolysis of copper sulfate the following electrode reactions occur Cathode: Cu 2+ + 2 e- Cu Copper is deposited and brown metal coats the electrode Anode: 4 OH- 2 H 2 O + O 2 + 4 e-

During the electrolysis of copper sulfate with graphite electrodes why is Cu formed at the cathode instead of H 2

Ions lower in the reactivity series are discharged (converted to atoms) in favour of ones above them

construct ionic half equations for the following electrolytes with graphite electrodes • Molten Pb. Br 2 • Concentrated aqueous Na. Cl • dilute aqueous Na. Cl • Concentrated aqueous Cu. SO 4 • Concentrated aqueous HCl • Dilute aqueous HCl • Molten magnesium chloride
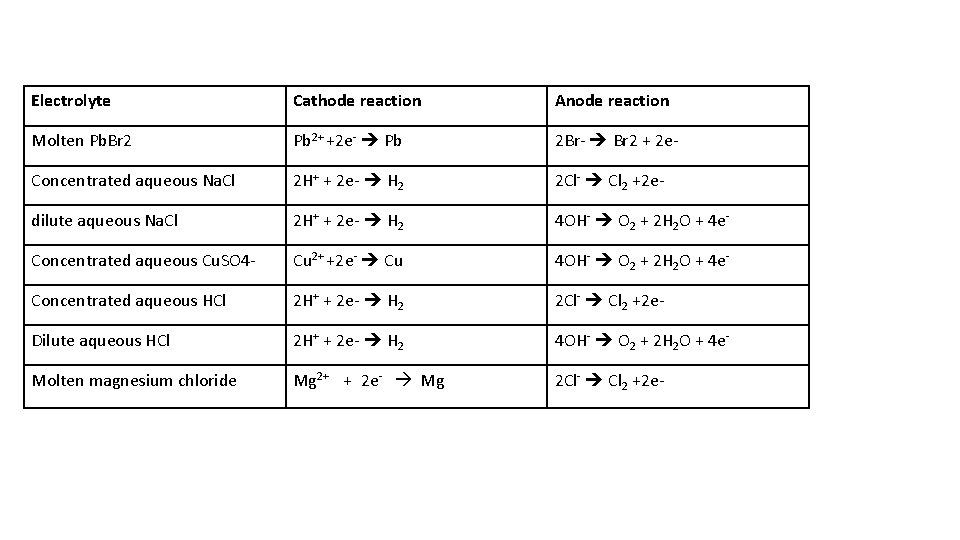
Electrolyte Cathode reaction Anode reaction Molten Pb. Br 2 Pb 2+ +2 e- Pb 2 Br- Br 2 + 2 e- Concentrated aqueous Na. Cl 2 H+ + 2 e- H 2 2 Cl- Cl 2 +2 e- dilute aqueous Na. Cl 2 H+ + 2 e- H 2 4 OH- O 2 + 2 H 2 O + 4 e- Concentrated aqueous Cu. SO 4 - Cu 2+ +2 e- Cu 4 OH- O 2 + 2 H 2 O + 4 e- Concentrated aqueous HCl 2 H+ + 2 e- H 2 2 Cl- Cl 2 +2 e- Dilute aqueous HCl 2 H+ + 2 e- H 2 4 OH- O 2 + 2 H 2 O + 4 e- Molten magnesium chloride Mg 2+ + 2 e- Mg 2 Cl- Cl 2 +2 e-

Explain why as electrolysis proceeds the aqueous copper(II) sulfate electrolyte becomes more acidic where as a sodium chloride electrolyte becomes more alkaline

copper(II) sulfate electrolyte : An aqueous solution of copper (II) sulphate contains four types of ions: • Ions from copper (II) sulphate: Cu 2+ and SO 42 • Ions from water: H+ and OHDuring electrolysis water and oxygen are formed at the anode and copper at the cathode H+ ions are left in solution so making it more acidic sodium chloride electrolyte : Ions from sodium chloride: Na+ and Cl. Ions from water: H+ and OHDuring electrolysis chlorine gas is formed at the anode and hydrogen gas at the cathode Na. OH is left in solution so making it more alkaline
What is meant by the term electroplating

Electroplating is the process of applying a thin layer of metal to another metal using electrolysis It can be used to add a more expensive metal to a cheaper one

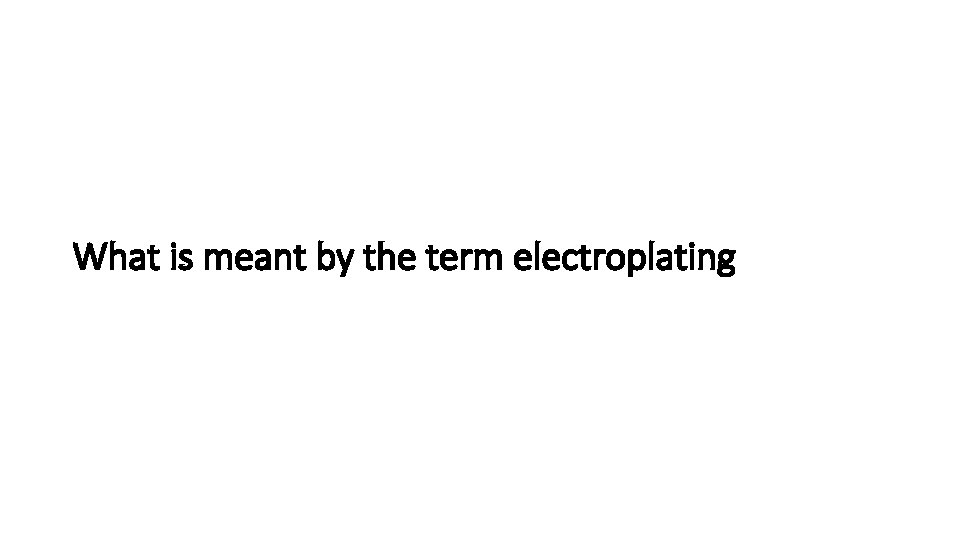
Describe the electroplating of metals
• The object to be electroplated is the cathode(-) • The metal to be coated onto the object is the anode(+). In this case silver • The electrolyte is a solution of a salt of the same metal that is being used for the anode. In this case silver nitrate solution Ag. NO 3 • The metal ions in the solution move towards the cathode and gain electrons eg. Ag+ + e- Ag • Coating the cathode

Give an advantage of electroplating

Prevents rusting Makes more attractive

Give a disadvantage of electroplating

Over time thin outer layer of metal can wear off and needs to be recoated

What is an ore?

An ore is a rock that contains enough of a metal to make it economical to extract

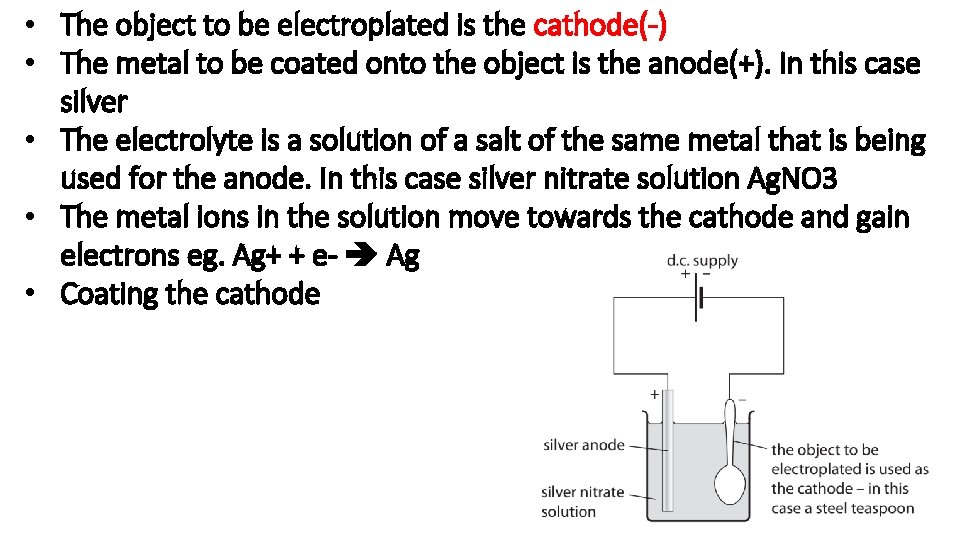
Describe how aluminium is extracted from aluminium ore
• Aluminium oxide , Al 2 O 3 is first melted • When molten the Al 3+ ions move to the cathode and pure aluminium is formed • The O 2 - moves to the anode where oxygen is formed

Why is cryolite added to aluminium oxide

• Aluminium oxides has a very high melting point of over 2000 o. C. • This is lowered to around 1000 o. C by adding cryolite • This saves a lot of energy in getting aluminium oxide molten

Why must the graphite electrode be constantly replaced in the electrolysis of aluminium oxide

• The oxygen that is formed at the anode reacts with carbon to form carbon dioxide • Therefore the anodes burn away and need to be replaced

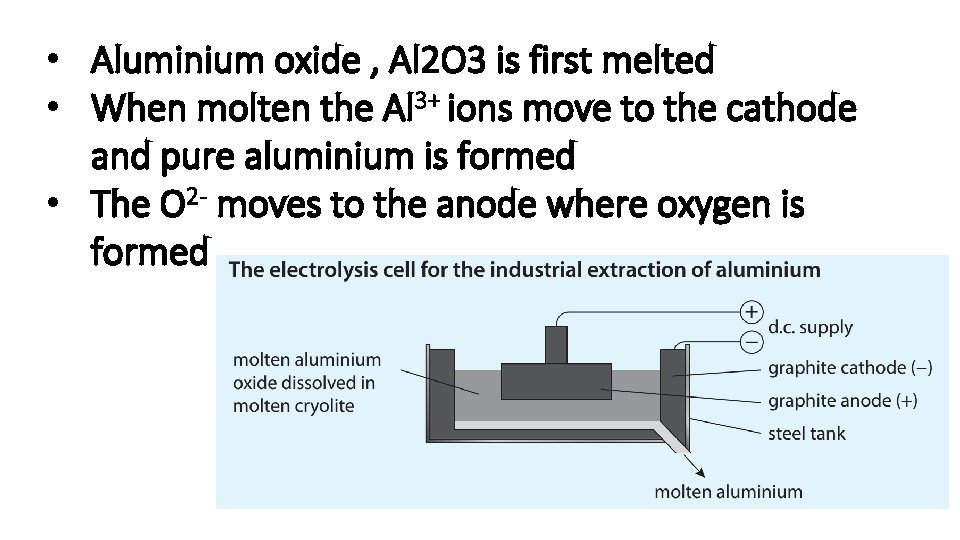
Describe the manufacture of chlorine, hydrogen and sodium hydroxide from concentrated aqueous sodium chloride (brine)
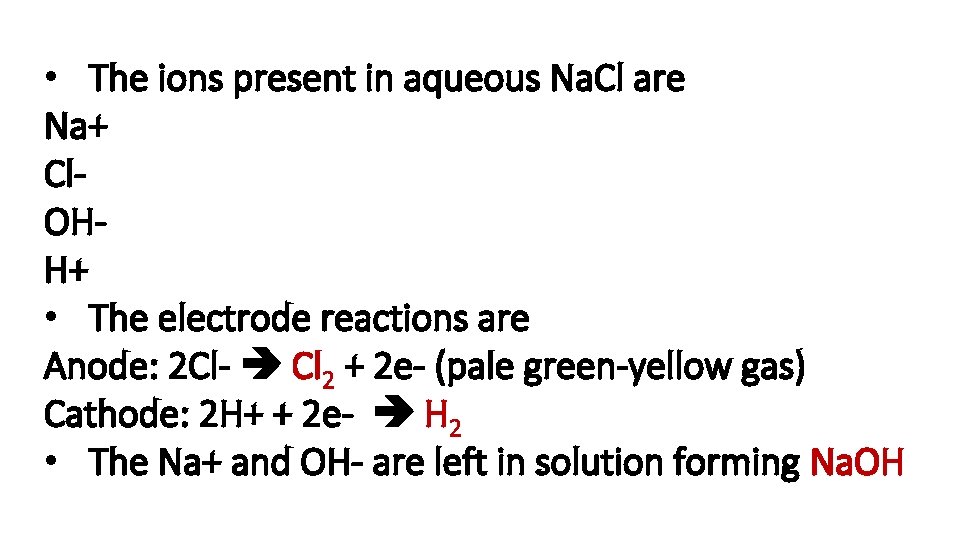
• The ions present in aqueous Na. Cl are Na+ Cl. OHH+ • The electrode reactions are Anode: 2 Cl- Cl 2 + 2 e- (pale green-yellow gas) Cathode: 2 H+ + 2 e- H 2 • The Na+ and OH- are left in solution forming Na. OH

• Describe how you could test for the preessence of hydrogen at the cathode

• Test: lighted splint • Result: squeaky pop

describe the production of energy from electrochemical cells

The more reactive a metal the easier it looses an electrons When two metals of different reactivity are dipped into an electrolyte they produce electrical energy and an electric current flows through the wire connecting them This is called a simple cell A cell converts chemical energy into electrical

Give an example of a simple cell
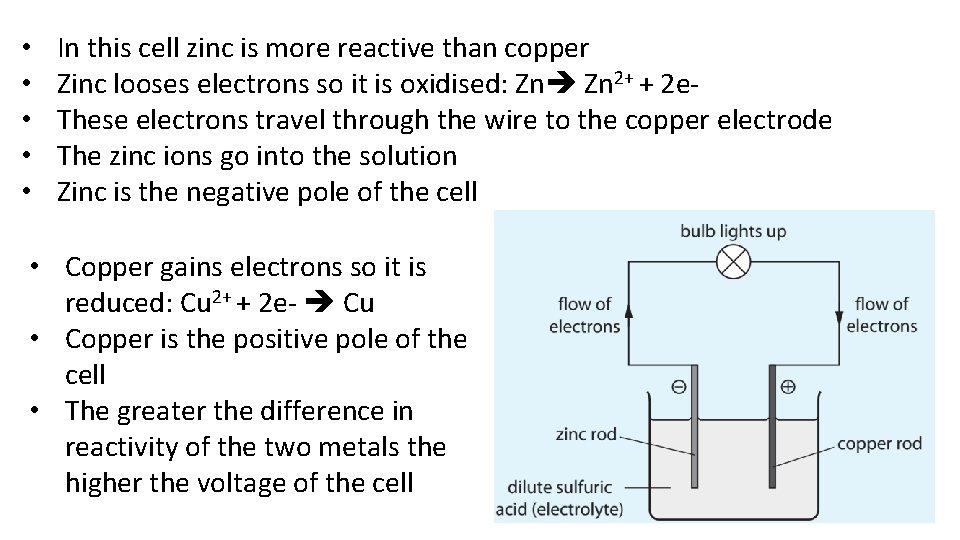
• • • In this cell zinc is more reactive than copper Zinc looses electrons so it is oxidised: Zn Zn 2+ + 2 e. These electrons travel through the wire to the copper electrode The zinc ions go into the solution Zinc is the negative pole of the cell • Copper gains electrons so it is reduced: Cu 2+ + 2 e- Cu • Copper is the positive pole of the cell • The greater the difference in reactivity of the two metals the higher the voltage of the cell
- Slides: 65