DBT 206 FONDRY TECHNOLOGY LECTURE WK 10 MELTING

DBT 206 FONDRY TECHNOLOGY LECTURE WK 10 MELTING TECHNIQUES & SOLIDIFICATION
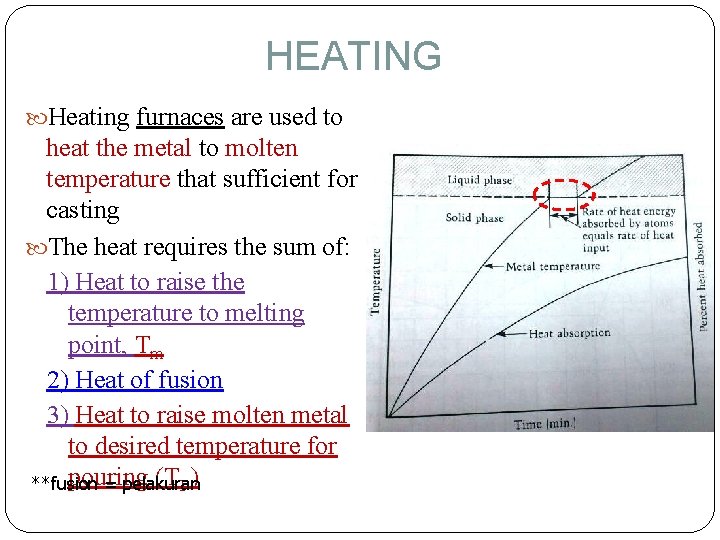
HEATING Heating furnaces are used to heat the metal to molten temperature that sufficient for casting The heat requires the sum of: 1) Heat to raise the temperature to melting point, Tm 2) Heat of fusion 3) Heat to raise molten metal to desired temperature for pouring (TP) **fusion = pelakuran
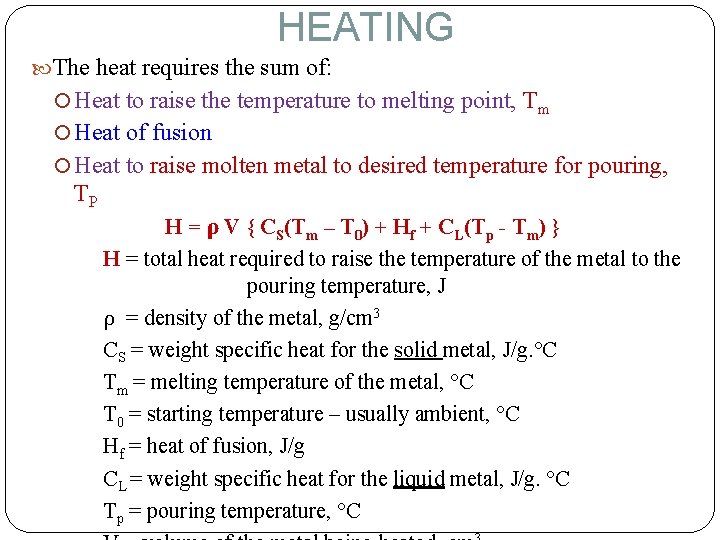
HEATING The heat requires the sum of: Heat to raise the temperature to melting point, Tm Heat of fusion Heat to raise molten metal to desired temperature for pouring, TP H = ρ V { CS(Tm – T 0) + Hf + CL(Tp - Tm) } H = total heat required to raise the temperature of the metal to the pouring temperature, J ρ = density of the metal, g/cm 3 CS = weight specific heat for the solid metal, J/g. °C Tm = melting temperature of the metal, °C T 0 = starting temperature – usually ambient, °C Hf = heat of fusion, J/g CL = weight specific heat for the liquid metal, J/g. °C Tp = pouring temperature, °C
Heating the metal 1 m 3 of a certain alloy is heated in a crucible from room temperature to 100°C above its melting point for casting. The alloy’s density is 7. 5 g/cm 3, Tm is 800°C, specific heat in solid state is 0. 33 J/g°C and 0. 29 J/g°C in liquid state, heat of fusion is 160 J/g. How much heat energy must be added to accomplish the heating?
MELTING Melting process of supplying necessary thermal energy to a solid material When metal is solid, atoms located in fixed position Higher the temperature, atoms move or diffuse within structure Melted when enough thermal energy, exceed residual energy level to overcome the strength of atomic bond When temperature , atoms more excited & increase speed more free to travel, tends to become homogeneous
MELTING Melting phenomenon: Increment of temperature rise Atoms move apart by force thermal energy As heating continues, temperature rise become slower In heating medium (e. g furnace) temperature gradient When metal reaches furnace temperature, temperature gradient getting less Temperature climbs stop Thermal energy still available but absorbed by atomic bonding force (crystallographic plane/grain boundaries) heat input = heat absorb = no temperature rise Finally the absorbed heat breaks the bond attraction & reach liquid phase
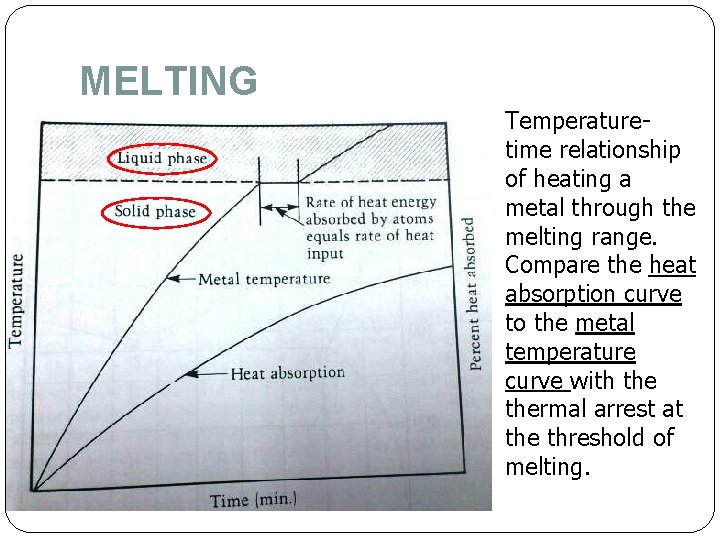
MELTING Temperaturetime relationship of heating a metal through the melting range. Compare the heat absorption curve to the metal temperature curve with thermal arrest at the threshold of melting.
Melting Techniques The metal obtained from the blast furnace, steel making furnace or other smelting furnaces (in case of non–ferrous metals) are NOT cast directly into the desired shapes of components. The reasons are: The metal obtained is not always in a sufficiently refined state to be directly cast. It is difficult to pour a huge quantity of molten metal in moulds in different sizes and shapes. The iron obtained from the smelting furnaces is cast first into some regular forms such as ingots & pig irons. These forms are re-melted in foundries for casting the required objects. For re-melting purposes, the equipments are; Crucible furnace Open-hearth furnace **pig iron = besi jongkong Cupola furnace Electric furnace

POURING After heating, the metal is ready for pouring Introduction of molten metal into the mold, including its flow through the gating system & into the cavity ais critical step in the casting process. For this step to be successful, the metal must flow into all regions of the mold before solidifying. Factors affecting the pouring operation include; 1) Pouring temperature (TP) 2) Pouring rate (cm 3/s) 3) Turbulence
POURING (1) Pouring temperature, TP -Definition: Temperature of molten metal the moment it is introduced into the mold - Superheat; temperature difference between pouring temperature & temperature at which freezing begins - or amount of heat that must be removed from molten metal between pouring & when solidification begins -TP influences metallographic structure, particularly grain size & grain structure. - increase temp, increase solubility of gas -to minimize defects in casting due to trapped gasses, TP should be kept low. -As the fluidity consideration & minimization of gas solubility are two requirements, the optimum temperature decided on the basis of fluidity requirements. (2) Pouring rate - Definition: Volumetric rate at which molten metal is poured into mold (cm 3/s) -Rate too slow; metal will chill & freeze before filling the cavity -Rate too fast; turbulence! (3) Turbulence -Definition: Erratic vibrations in magnitude & direction of velocity throughout the fluid -Must be avoided! Because. . (1) Accelerate formation of metal oxides – degrade cast product quality (2) mold erosion – wear of the mold surfaces due to impact of the flowing molten metal
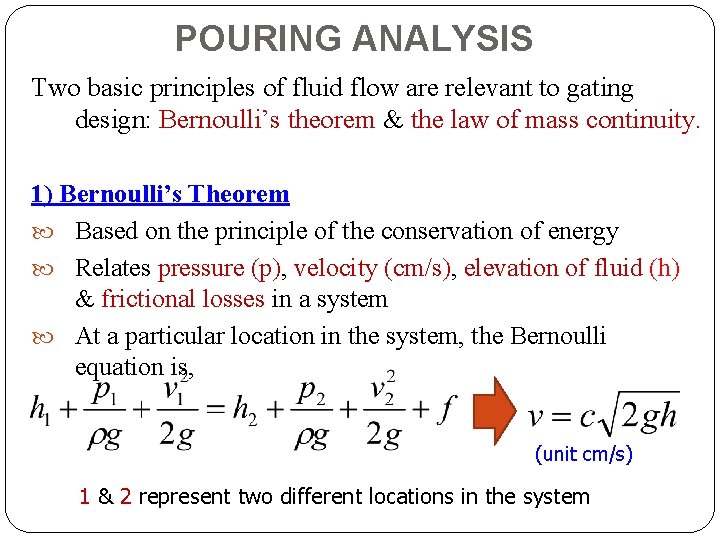
POURING ANALYSIS Two basic principles of fluid flow are relevant to gating design: Bernoulli’s theorem & the law of mass continuity. 1) Bernoulli’s Theorem Based on the principle of the conservation of energy Relates pressure (p), velocity (cm/s), elevation of fluid (h) & frictional losses in a system At a particular location in the system, the Bernoulli equation is, (unit cm/s) 1 & 2 represent two different locations in the system
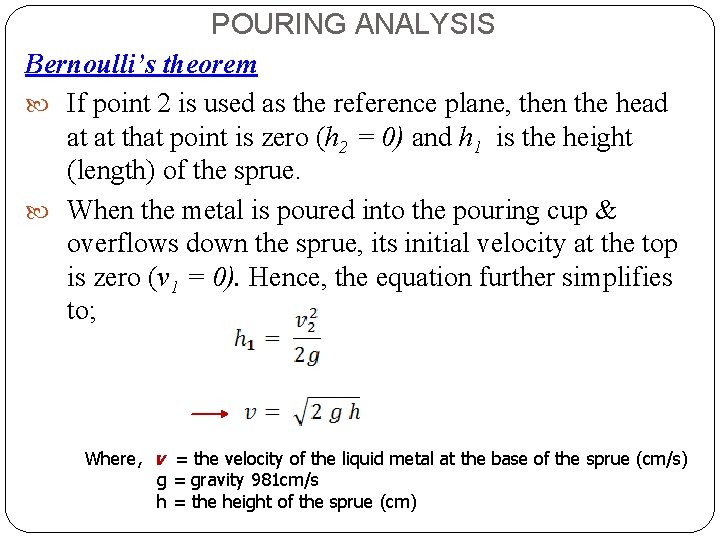
POURING ANALYSIS Bernoulli’s theorem If point 2 is used as the reference plane, then the head at at that point is zero (h 2 = 0) and h 1 is the height (length) of the sprue. When the metal is poured into the pouring cup & overflows down the sprue, its initial velocity at the top is zero (v 1 = 0). Hence, the equation further simplifies to; Where, v = the velocity of the liquid metal at the base of the sprue (cm/s) g = gravity 981 cm/s h = the height of the sprue (cm)
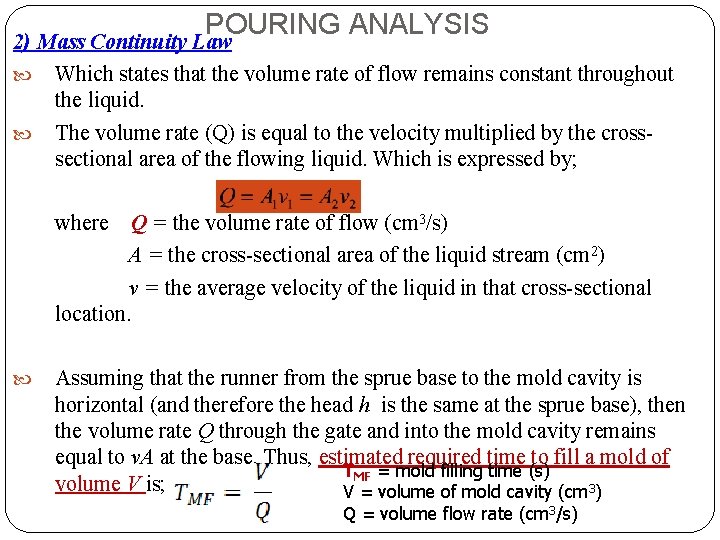
POURING ANALYSIS 2) Mass Continuity Law Which states that the volume rate of flow remains constant throughout the liquid. The volume rate (Q) is equal to the velocity multiplied by the crosssectional area of the flowing liquid. Which is expressed by; where Q = the volume rate of flow (cm 3/s) A = the cross-sectional area of the liquid stream (cm 2) v = the average velocity of the liquid in that cross-sectional location. Assuming that the runner from the sprue base to the mold cavity is horizontal (and therefore the head h is the same at the sprue base), then the volume rate Q through the gate and into the mold cavity remains equal to v. A at the base. Thus, estimated required time to fill a mold of TMF = mold filling time (s) volume V is; V = volume of mold cavity (cm 3) Q = volume flow rate (cm 3/s)
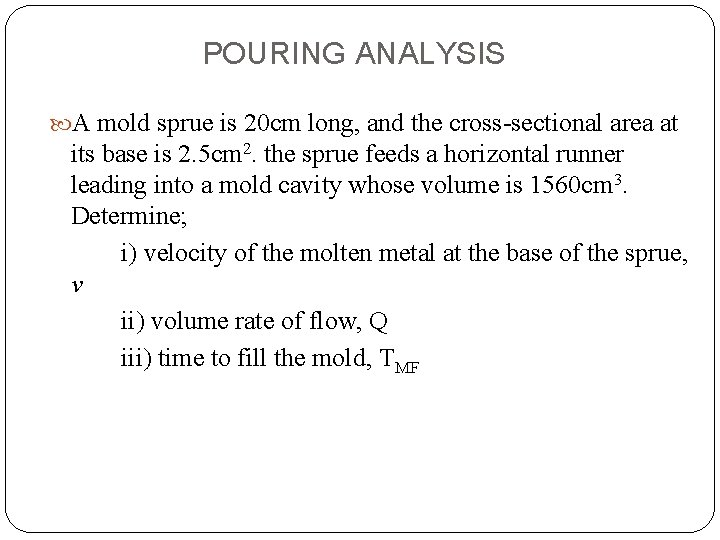
POURING ANALYSIS A mold sprue is 20 cm long, and the cross-sectional area at its base is 2. 5 cm 2. the sprue feeds a horizontal runner leading into a mold cavity whose volume is 1560 cm 3. Determine; i) velocity of the molten metal at the base of the sprue, v ii) volume rate of flow, Q iii) time to fill the mold, TMF
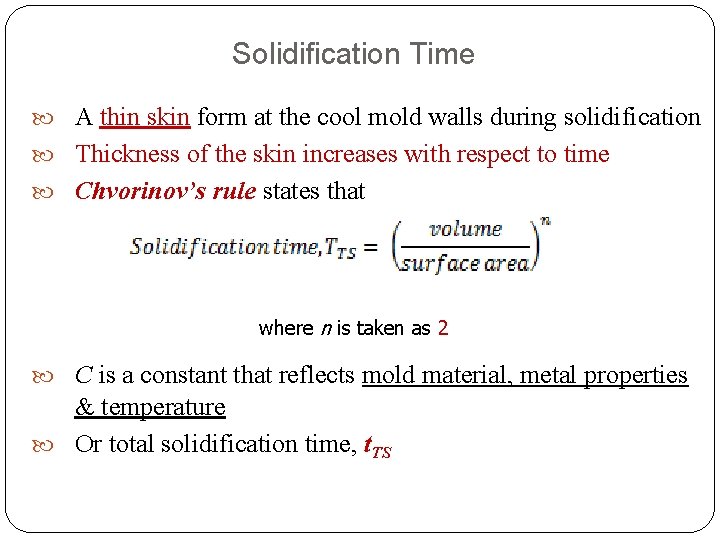
Solidification Time A thin skin form at the cool mold walls during solidification Thickness of the skin increases with respect to time Chvorinov’s rule states that where n is taken as 2 C is a constant that reflects mold material, metal properties & temperature Or total solidification time, t. TS
Solidification of Metals: Alloys Effects of Cooling Rates Slow cooling rates coarse dendritic structures with large spacing between dendrite arms Higher (faster) cooling rates structure becomes finer with smaller dendrite arm spacing Smaller the grain size of the cast alloy; strength ductility microporosity tendency for casting to crack
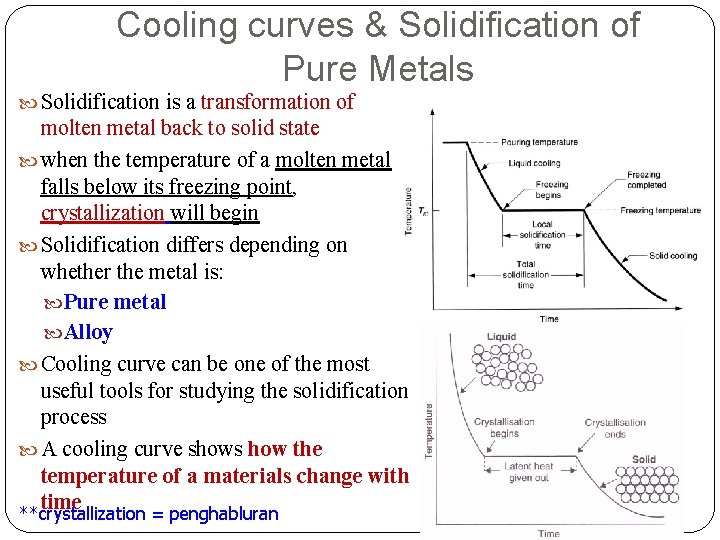
Cooling curves & Solidification of Pure Metals Solidification is a transformation of molten metal back to solid state when the temperature of a molten metal falls below its freezing point, crystallization will begin Solidification differs depending on whether the metal is: Pure metal Alloy Cooling curve can be one of the most useful tools for studying the solidification process A cooling curve shows how the temperature of a materials change with time **crystallization = penghabluran
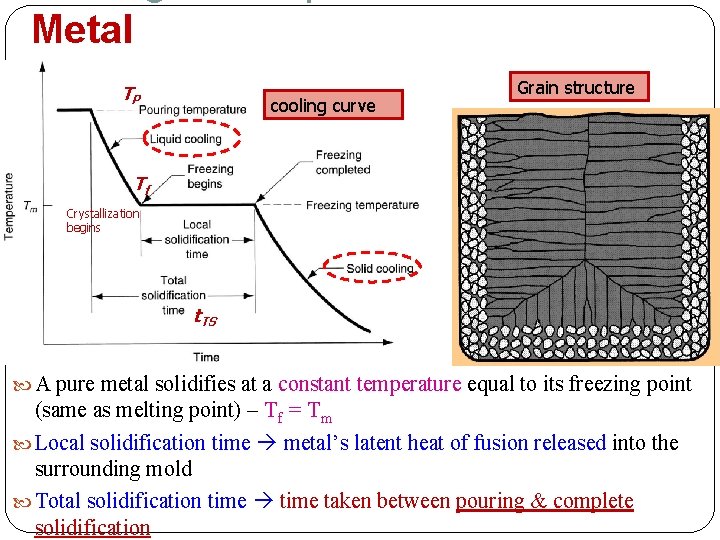
Metal TP cooling curve Grain structure Tf Crystallization begins t. TS A pure metal solidifies at a constant temperature equal to its freezing point (same as melting point) – Tf = Tm Local solidification time metal’s latent heat of fusion released into the surrounding mold Total solidification time taken between pouring & complete solidification
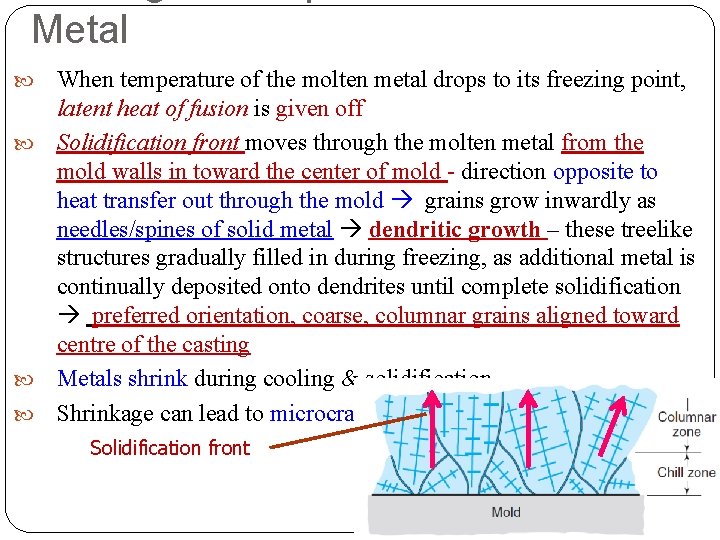
Metal When temperature of the molten metal drops to its freezing point, latent heat of fusion is given off Solidification front moves through the molten metal from the mold walls in toward the center of mold - direction opposite to heat transfer out through the mold grains grow inwardly as needles/spines of solid metal dendritic growth – these treelike structures gradually filled in during freezing, as additional metal is continually deposited onto dendrites until complete solidification preferred orientation, coarse, columnar grains aligned toward centre of the casting Metals shrink during cooling & solidification Shrinkage can lead to microcracking & associated porosity Solidification front
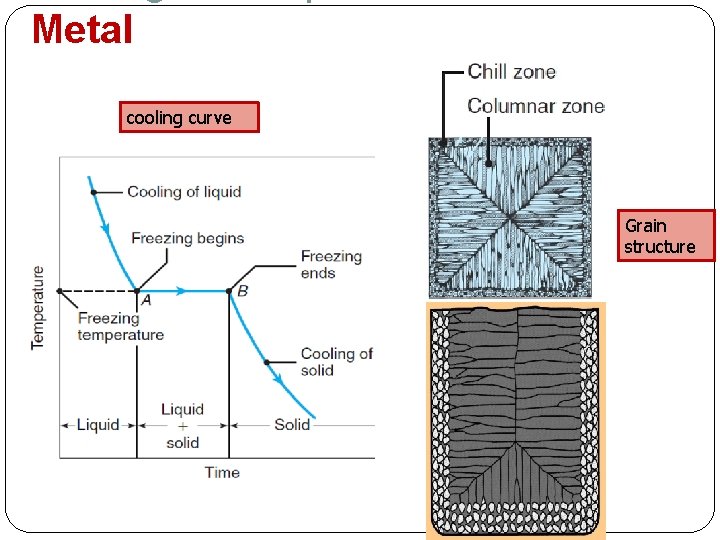
Metal cooling curve Grain structure
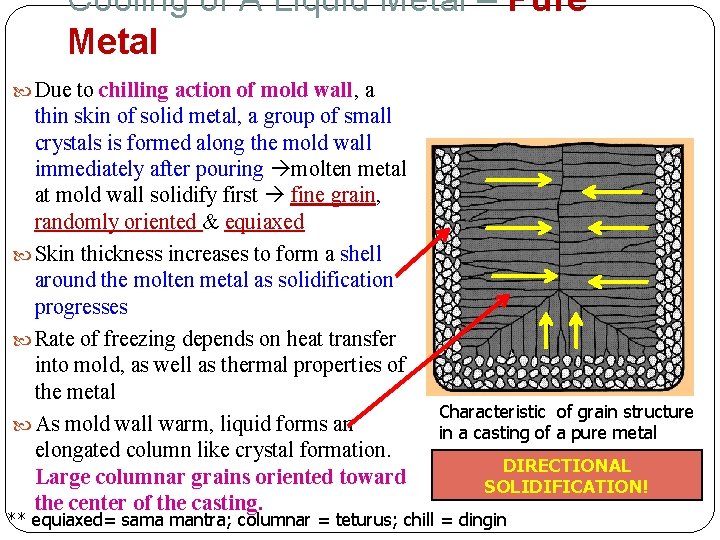
Cooling of A Liquid Metal – Pure Metal Due to chilling action of mold wall, a thin skin of solid metal, a group of small crystals is formed along the mold wall immediately after pouring molten metal at mold wall solidify first fine grain, randomly oriented & equiaxed Skin thickness increases to form a shell around the molten metal as solidification progresses Rate of freezing depends on heat transfer into mold, as well as thermal properties of the metal As mold wall warm, liquid forms an elongated column like crystal formation. Large columnar grains oriented toward the center of the casting. Characteristic of grain structure in a casting of a pure metal DIRECTIONAL SOLIDIFICATION! ** equiaxed= sama mantra; columnar = teturus; chill = dingin
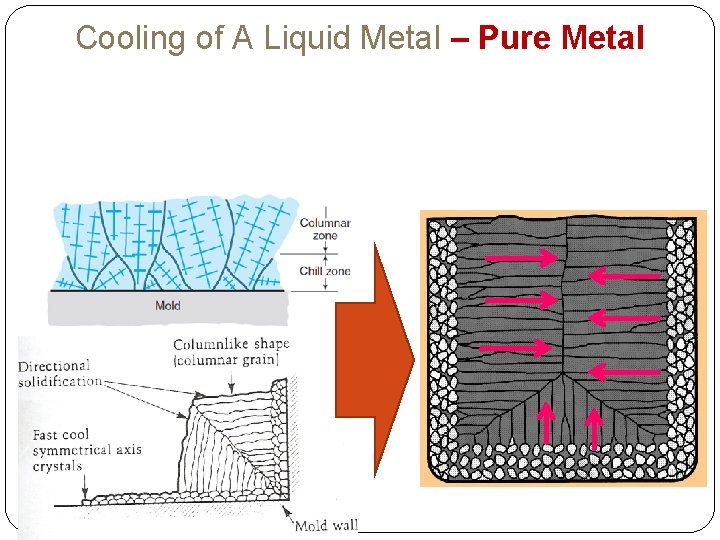
Cooling of A Liquid Metal – Pure Metal
Directional solidification In directional solidification, the solidification continues progressively from the thinnest section which solidifies first towards the risers which should be last to solidify Directional solidification can be ensured by; Designing & positioning the gating system & risers properly Increasing the thickness of certain sections of the casting Using exothermic materials in the risers Using chills in the moulds
Directional solidification Chills – internal or external heat sinks that cause rapid freezing in certain regions of the casting Internal chills are small metals parts placed inside the cavity before pouring so that the molten metal will solidify first around these parts The internal chill should have a chemical composition that is approximately the same as the metal being poured
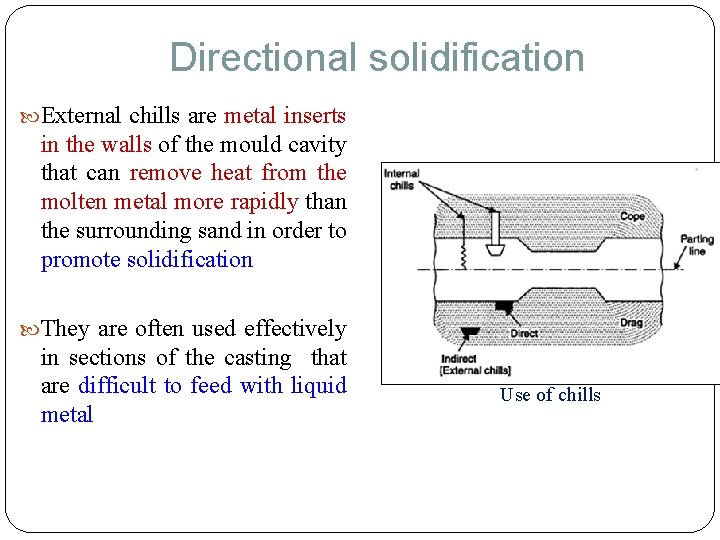
Directional solidification External chills are metal inserts in the walls of the mould cavity that can remove heat from the molten metal more rapidly than the surrounding sand in order to promote solidification They are often used effectively in sections of the casting that are difficult to feed with liquid metal Use of chills
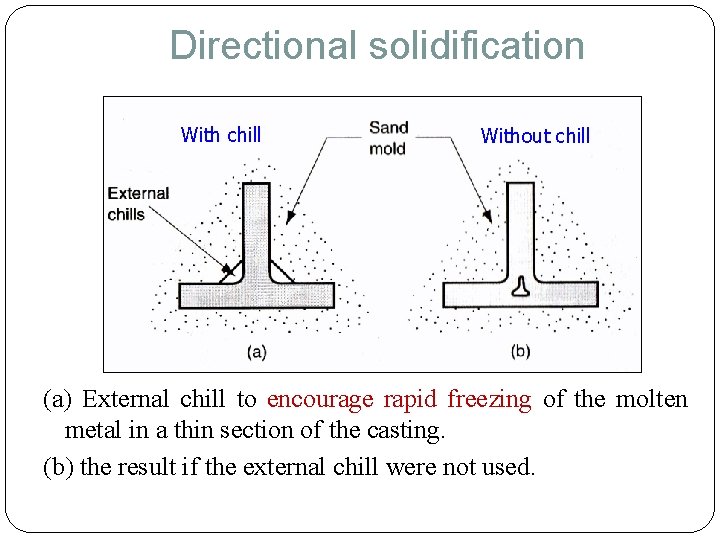
Directional solidification With chill Without chill (a) External chill to encourage rapid freezing of the molten metal in a thin section of the casting. (b) the result if the external chill were not used.
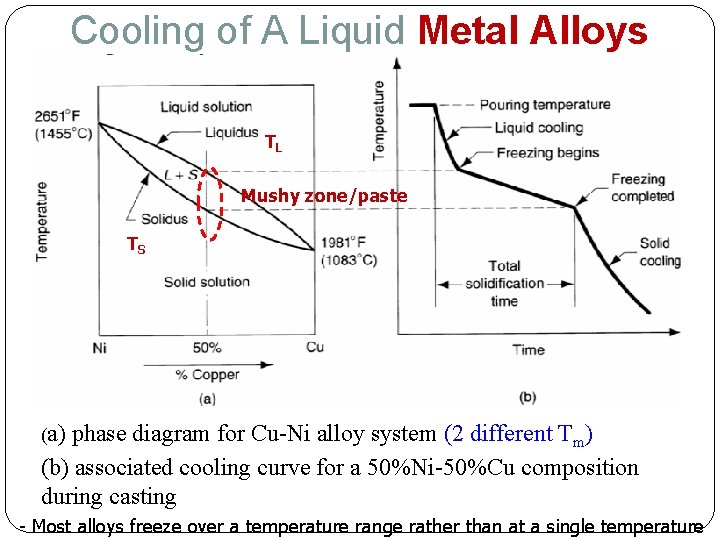
Cooling of A Liquid Metal Alloys TL Mushy zone/paste TS (a) phase diagram for Cu-Ni alloy system (2 different Tm) (b) associated cooling curve for a 50%Ni-50%Cu composition during casting - Most alloys freeze over a temperature range rather than at a single temperature
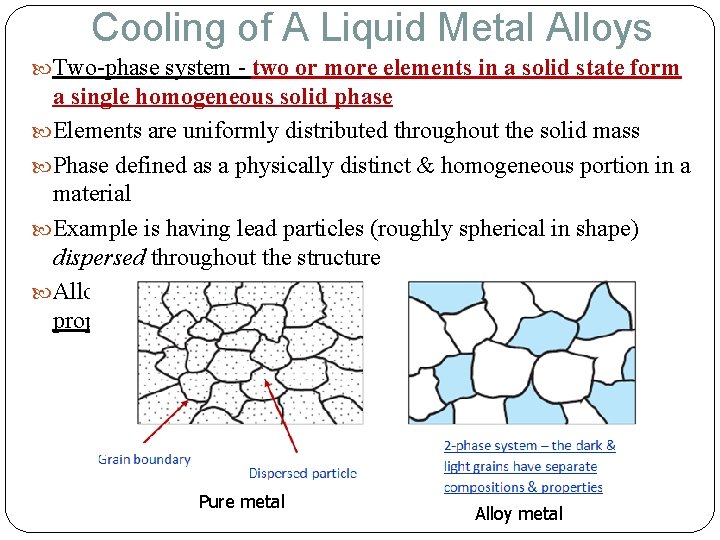
Cooling of A Liquid Metal Alloys Two-phase system - two or more elements in a solid state form a single homogeneous solid phase Elements are uniformly distributed throughout the solid mass Phase defined as a physically distinct & homogeneous portion in a material Example is having lead particles (roughly spherical in shape) dispersed throughout the structure Alloying is to strengthening metals alloys & controlling their properties Pure metal Alloy metal
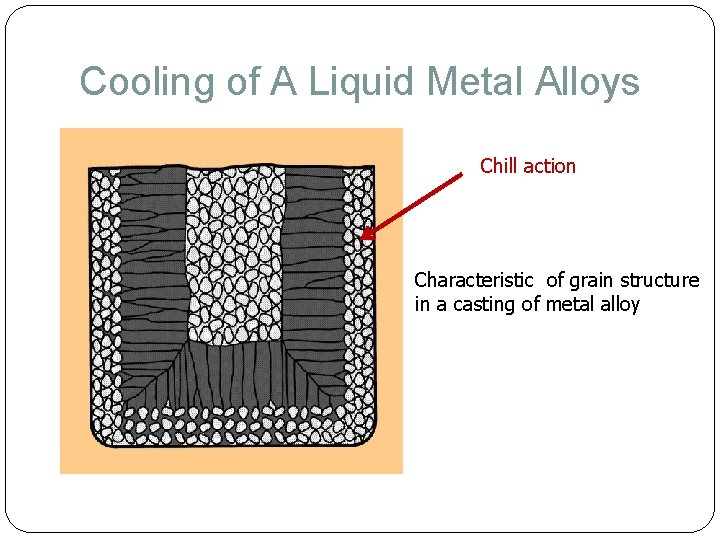
Cooling of A Liquid Metal Alloys Chill action Characteristic of grain structure in a casting of metal alloy
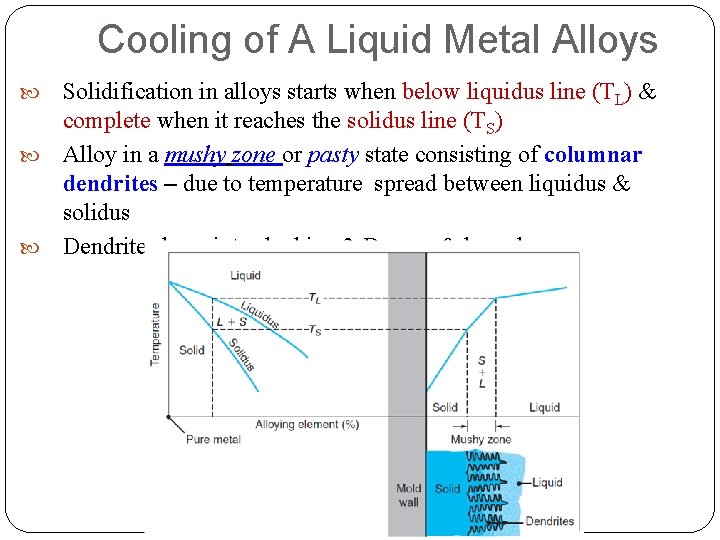
Cooling of A Liquid Metal Alloys Solidification in alloys starts when below liquidus line (TL) & complete when it reaches the solidus line (TS) Alloy in a mushy zone or pasty state consisting of columnar dendrites – due to temperature spread between liquidus & solidus Dendrites have inter-locking 3 -D arms & branches
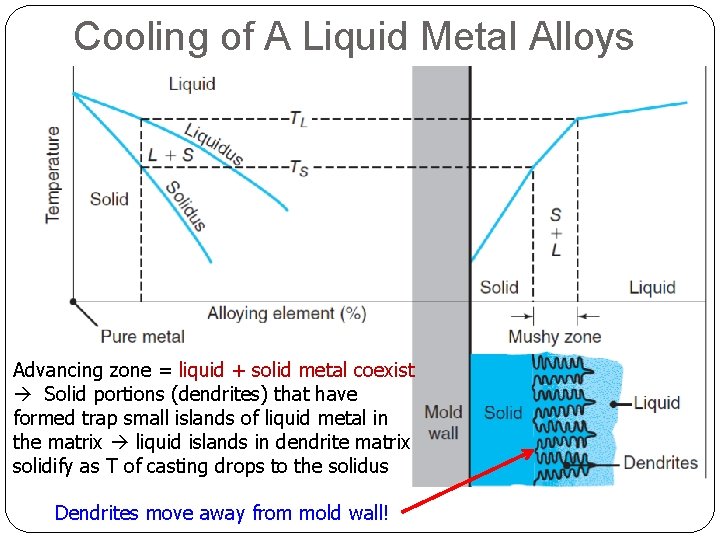
Cooling of A Liquid Metal Alloys Advancing zone = liquid + solid metal coexist Solid portions (dendrites) that have formed trap small islands of liquid metal in the matrix liquid islands in dendrite matrix solidify as T of casting drops to the solidus Dendrites move away from mold wall!
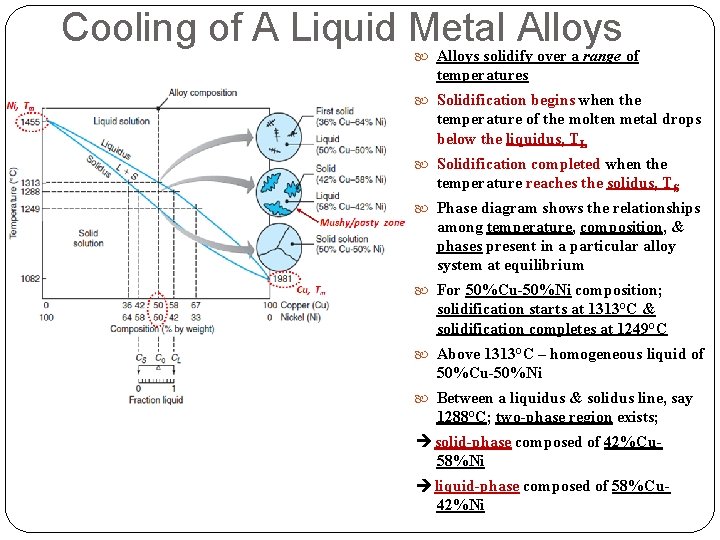
Cooling of A Liquid Metal Alloys solidify over a range of temperatures Solidification begins when the temperature of the molten metal drops below the liquidus, TL Solidification completed when the temperature reaches the solidus, TS Phase diagram shows the relationships among temperature, composition, & phases present in a particular alloy system at equilibrium For 50%Cu-50%Ni composition; solidification starts at 1313°C & solidification completes at 1249°C Above 1313°C – homogeneous liquid of 50%Cu-50%Ni Between a liquidus & solidus line, say 1288°C; two-phase region exists; solid-phase composed of 42%Cu 58%Ni liquid-phase composed of 58%Cu 42%Ni
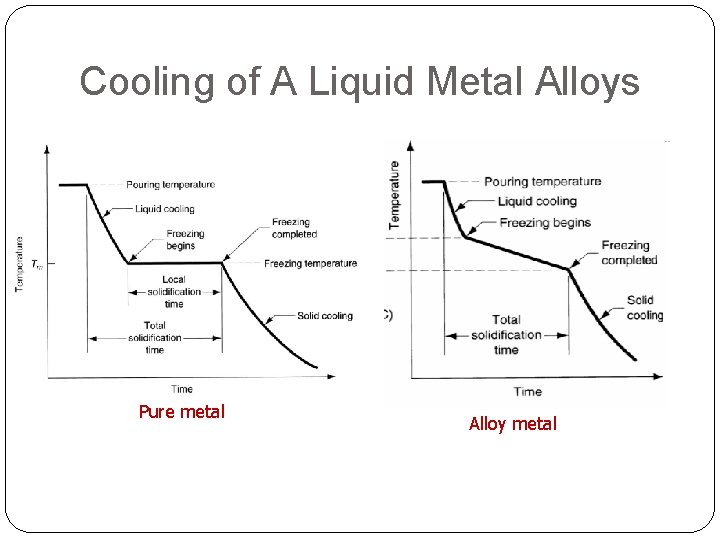
Cooling of A Liquid Metal Alloys Pure metal Alloy metal
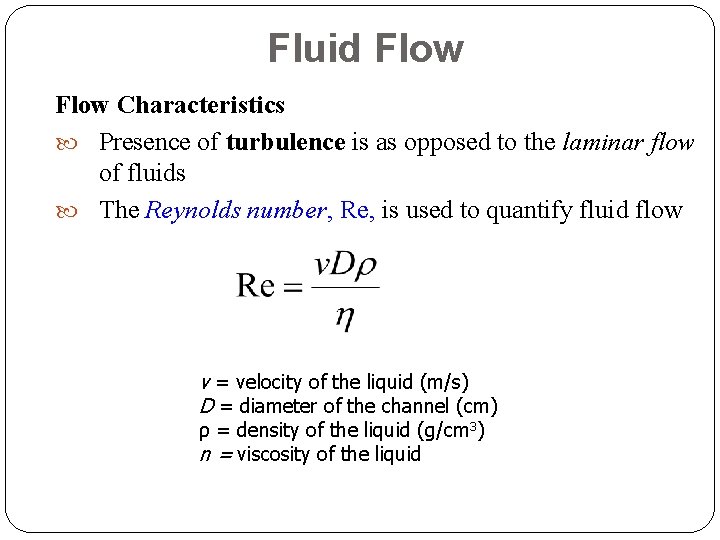
Fluid Flow Characteristics Presence of turbulence is as opposed to the laminar flow of fluids The Reynolds number, Re, is used to quantify fluid flow v = velocity of the liquid (m/s) D = diameter of the channel (cm) ρ = density of the liquid (g/cm 3) n = viscosity of the liquid
Fluidity of Molten Metal Fluidity - Ability of a metal to flow into & fill the mould before freezing Inverse of viscosity– viscosity increase, fluidity decrease Fluidity consists of 2 basic factors: 1. Characteristics of the molten metal 2. Casting parameters
Fluidity of Molten Metal Viscosity and viscosity index increase, fluidity decreases Surface Tension High surface tension of the liquid metal reduces fluidity Inclusions can have a adverse effect on fluidity Solidification Pattern of the Alloy Fluidity is inversely proportional to the freezing range Mold Design and dimensions of the sprue, runners and risers influence fluidity
Fluidity of Molten Metal Mold Material and its Surface Characteristics High thermal conductivity of the mold & the rough surfaces lower the fluidity Degree of Superheat(pouring T relative to Tm) Superheat improves fluidity by delaying solidification Rate of Pouring Slow rate of pouring lower the fluidity
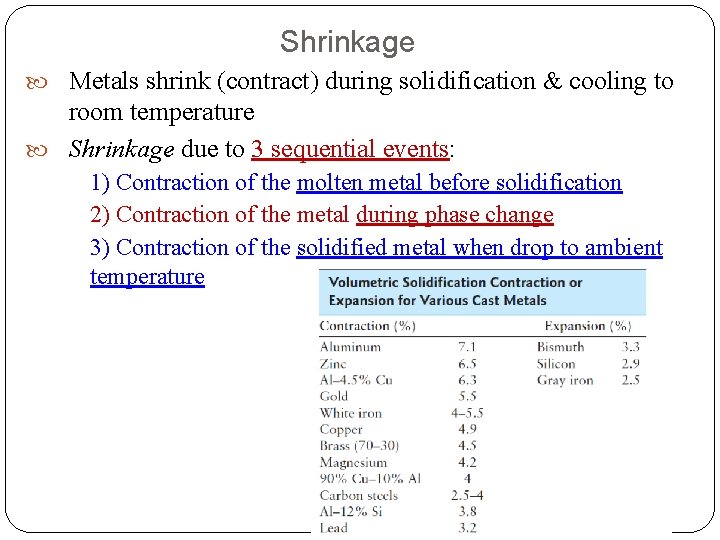
Shrinkage Metals shrink (contract) during solidification & cooling to room temperature Shrinkage due to 3 sequential events: 1) Contraction of the molten metal before solidification 2) Contraction of the metal during phase change 3) Contraction of the solidified metal when drop to ambient temperature
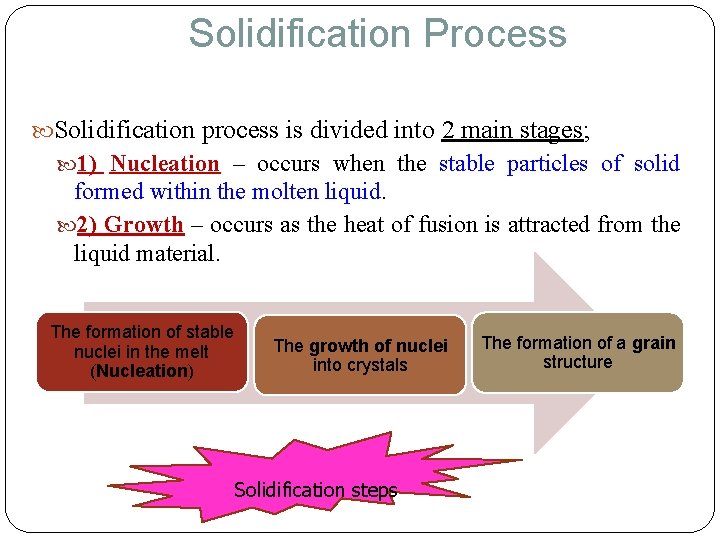
Solidification Process Solidification process is divided into 2 main stages; 1) Nucleation – occurs when the stable particles of solid formed within the molten liquid. 2) Growth – occurs as the heat of fusion is attracted from the liquid material. The formation of stable nuclei in the melt (Nucleation) The growth of nuclei into crystals Solidification steps The formation of a grain structure
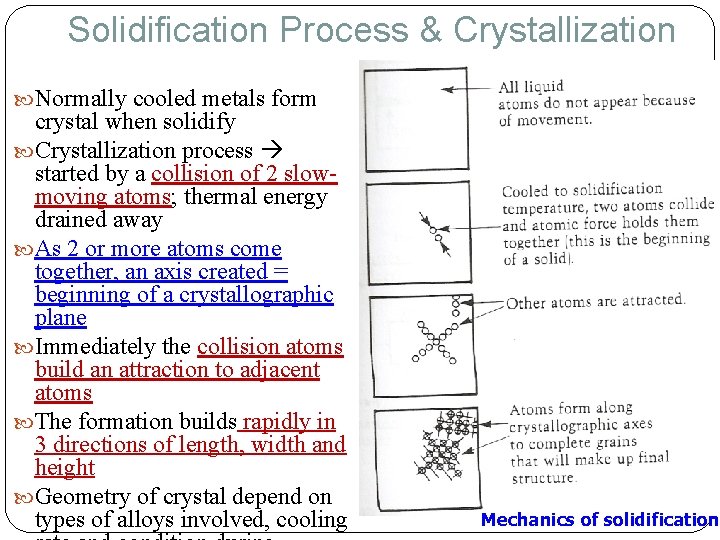
Solidification Process & Crystallization Normally cooled metals form crystal when solidify Crystallization process started by a collision of 2 slowmoving atoms; thermal energy drained away As 2 or more atoms come together, an axis created = beginning of a crystallographic plane Immediately the collision atoms build an attraction to adjacent atoms The formation builds rapidly in 3 directions of length, width and height Geometry of crystal depend on types of alloys involved, cooling Mechanics of solidification
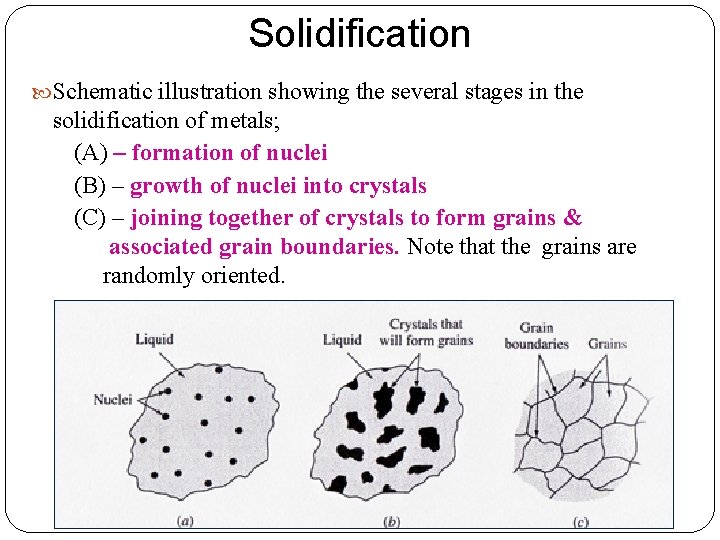
Solidification Schematic illustration showing the several stages in the solidification of metals; (A) – formation of nuclei (B) – growth of nuclei into crystals (C) – joining together of crystals to form grains & associated grain boundaries. Note that the grains are randomly oriented.
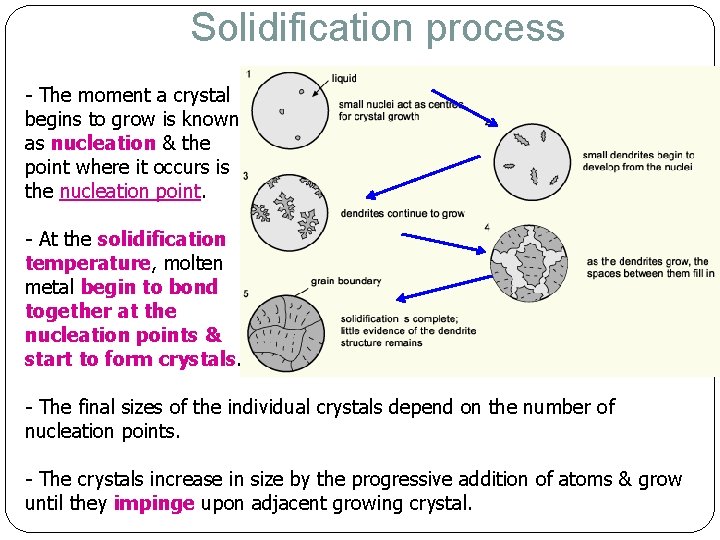
Solidification process - The moment a crystal begins to grow is known as nucleation & the point where it occurs is the nucleation point. - At the solidification temperature, molten metal begin to bond together at the nucleation points & start to form crystals. - The final sizes of the individual crystals depend on the number of nucleation points. - The crystals increase in size by the progressive addition of atoms & grow until they impinge upon adjacent growing crystal.
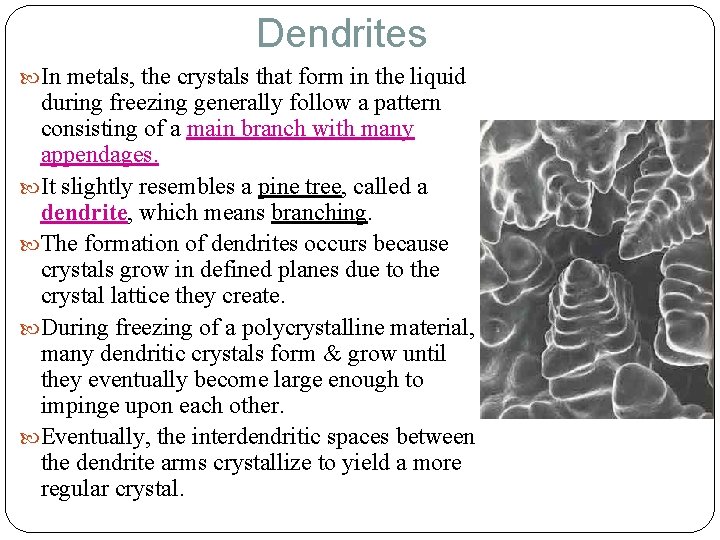
Dendrites In metals, the crystals that form in the liquid during freezing generally follow a pattern consisting of a main branch with many appendages. It slightly resembles a pine tree, called a dendrite, which means branching. The formation of dendrites occurs because crystals grow in defined planes due to the crystal lattice they create. During freezing of a polycrystalline material, many dendritic crystals form & grow until they eventually become large enough to impinge upon each other. Eventually, the interdendritic spaces between the dendrite arms crystallize to yield a more regular crystal.
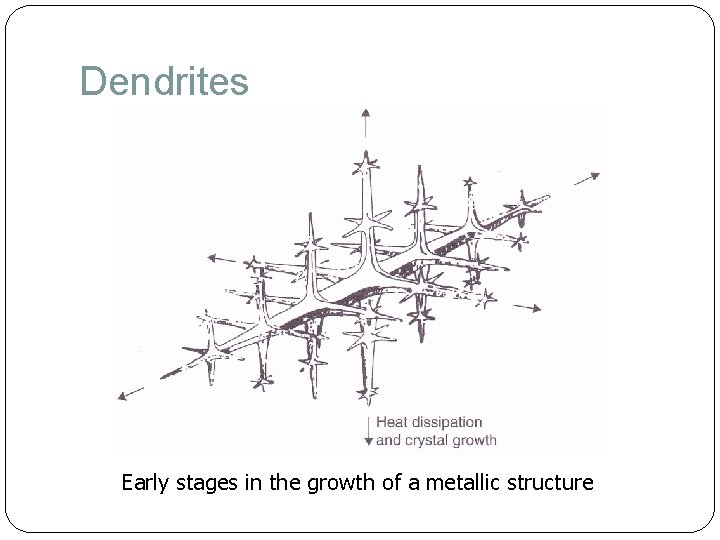
Dendrites Early stages in the growth of a metallic structure
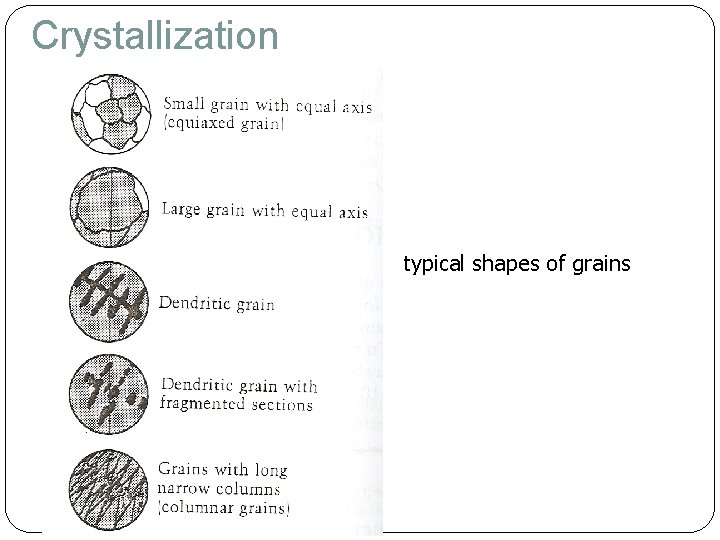
Crystallization typical shapes of grains

Thank You …Q & A session…
- Slides: 46