Day 27 Electron Configuration ELECTRON CONFIGURATION n n








- Slides: 27

Day 27 Electron Configuration

ELECTRON CONFIGURATION n n The arrangement of electrons in an atom is known as the atom’s electron configuration Just like each element has a unique atomic number, each has its own configuration, too.

The “ Theoretical Orbital” n n n Represents the space where an electron is located 90% of the time Represents the average distance an electron is from the nucleus Represents the shape of the traveling path of an electron

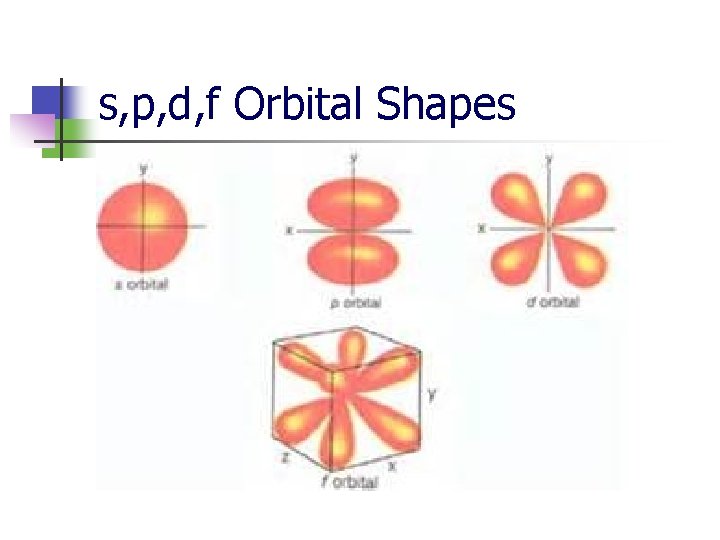
s, p, d, f Orbital Shapes

3 General Rules 1. 2. 3. The Aufbau Principle Hund’s Rule Pauli Exclusion Principle

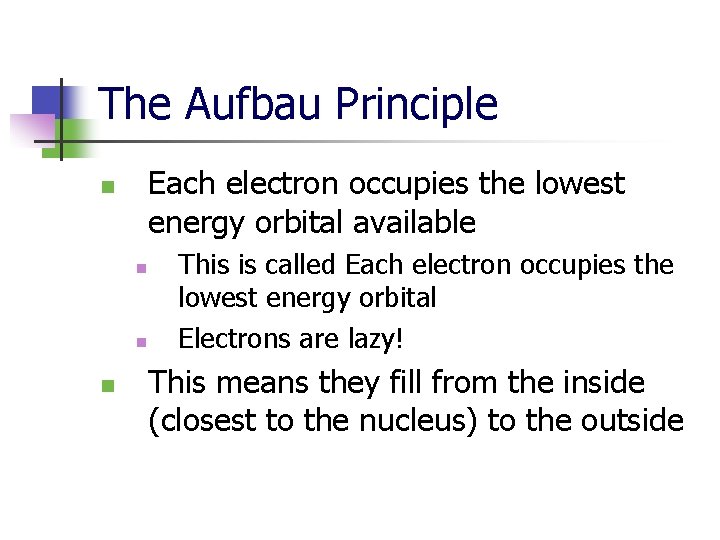
The Aufbau Principle Each electron occupies the lowest energy orbital available n n This is called Each electron occupies the lowest energy orbital Electrons are lazy! This means they fill from the inside (closest to the nucleus) to the outside

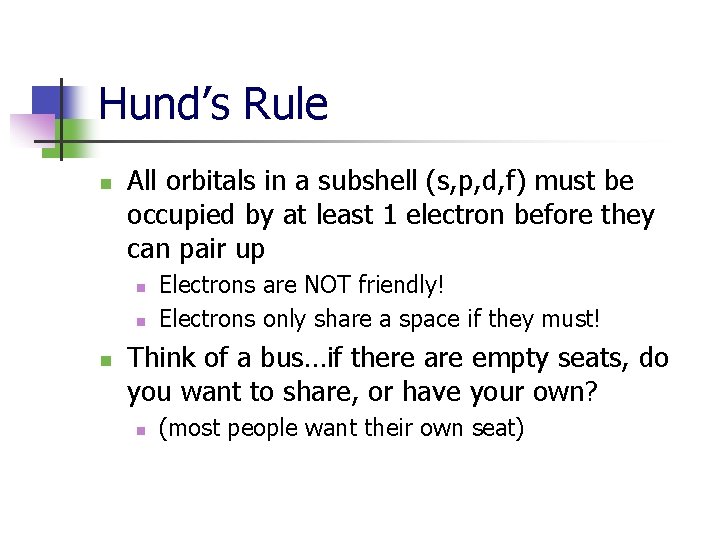
Hund’s Rule n All orbitals in a subshell (s, p, d, f) must be occupied by at least 1 electron before they can pair up n n n Electrons are NOT friendly! Electrons only share a space if they must! Think of a bus…if there are empty seats, do you want to share, or have your own? n (most people want their own seat)

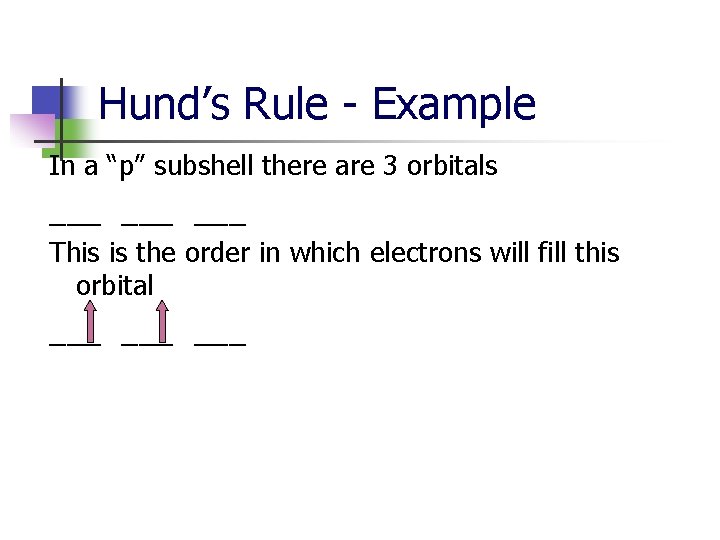
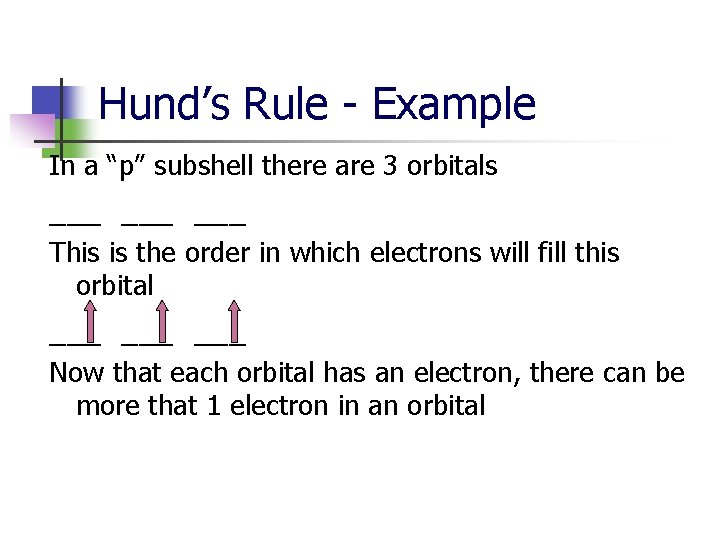
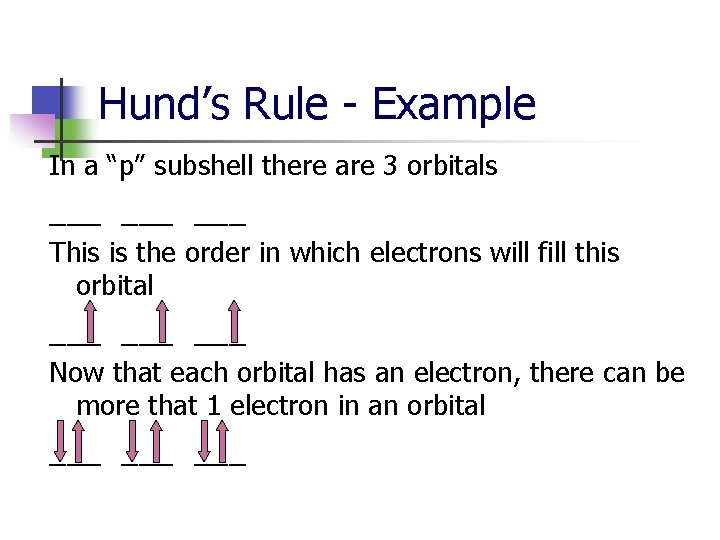
Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___

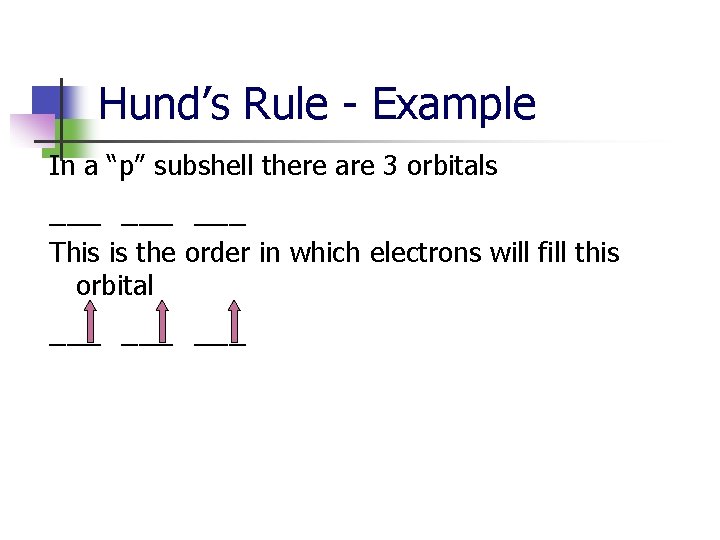
Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___

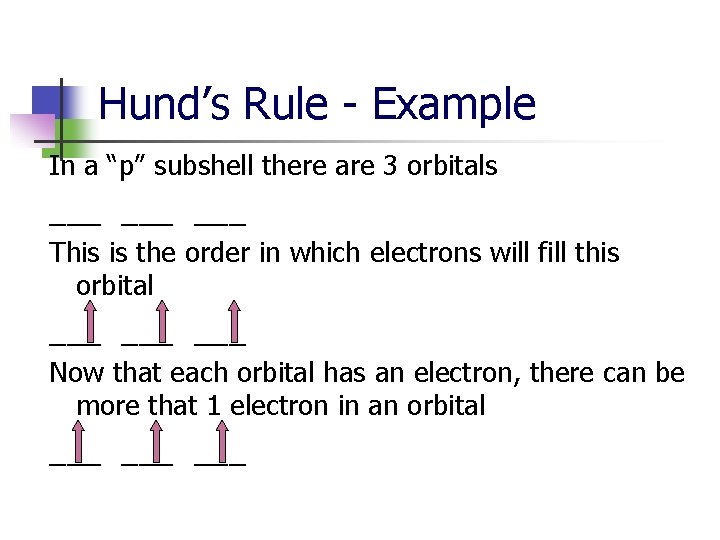
Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___

Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___ Now that each orbital has an electron, there can be more that 1 electron in an orbital

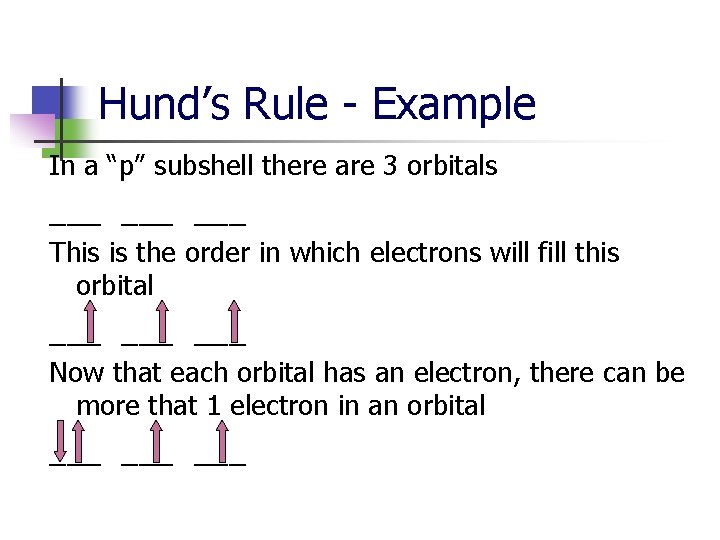
Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___ Now that each orbital has an electron, there can be more that 1 electron in an orbital ___ ___

Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___ Now that each orbital has an electron, there can be more that 1 electron in an orbital ___ ___

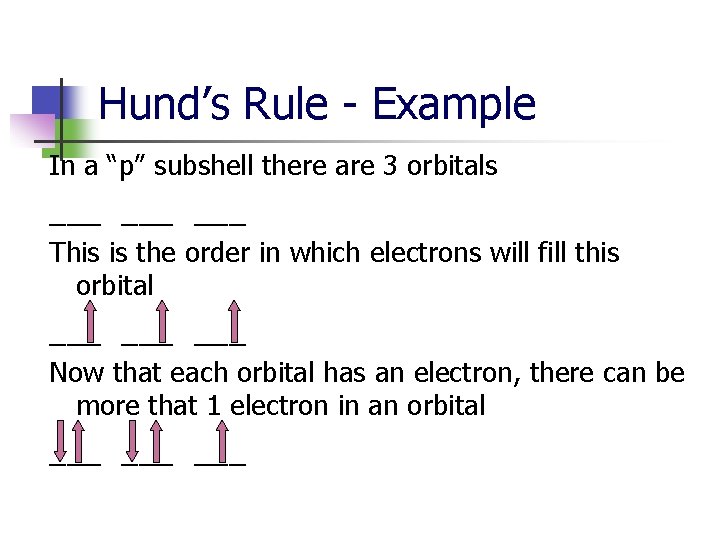
Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___ Now that each orbital has an electron, there can be more that 1 electron in an orbital ___ ___

Hund’s Rule - Example In a “p” subshell there are 3 orbitals ___ ___ This is the order in which electrons will fill this orbital ___ ___ Now that each orbital has an electron, there can be more that 1 electron in an orbital ___ ___

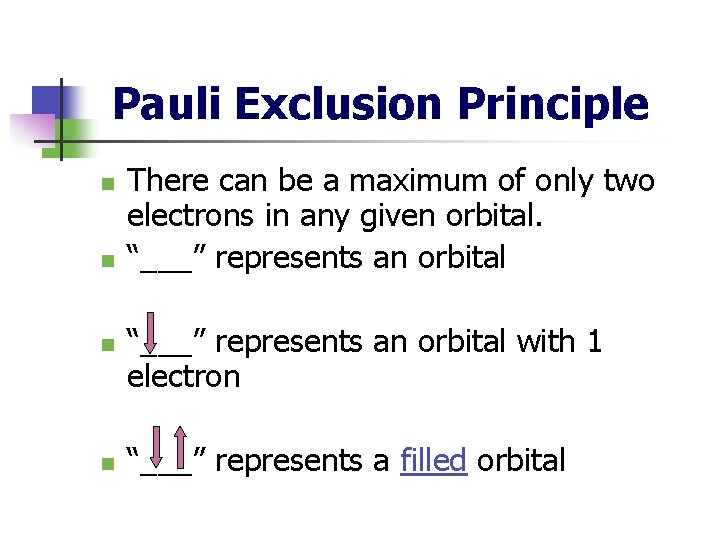
Pauli Exclusion Principle n n There can be a maximum of only two electrons in any given orbital. “___” represents an orbital with 1 electron “___” represents a filled orbital

Parts of an electron configuration: n n n Energy level - a number (1, 2, 3 and so on) Sublevel (orbital) - a letter, either s, p, d, or f Number of electrons - a superscript number

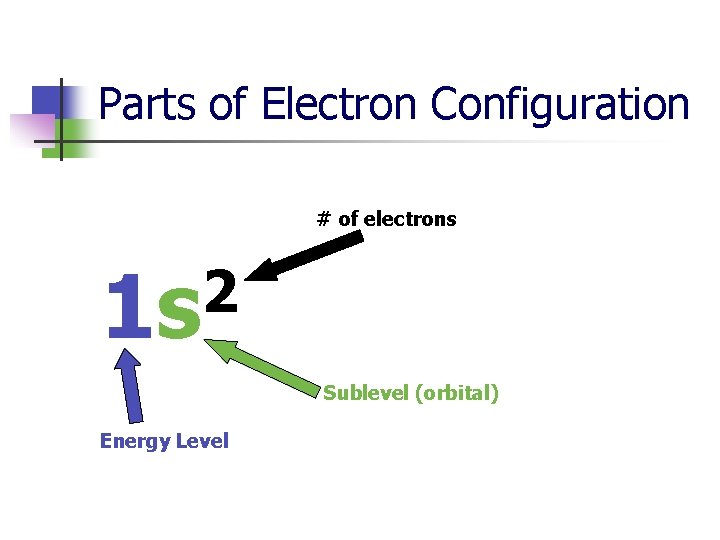
Parts of Electron Configuration # of electrons 2 1 s Sublevel (orbital) Energy Level

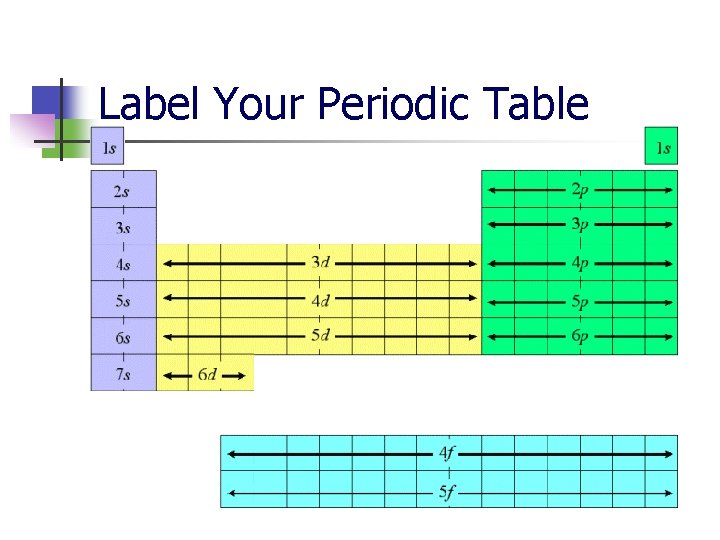
Label Your Periodic Table

Now Let’s Try a Few

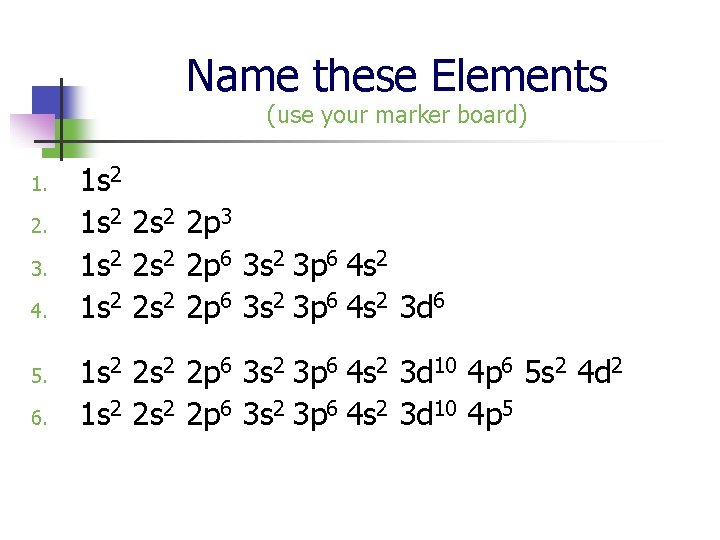
Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 2 s 2 2 p 3 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5

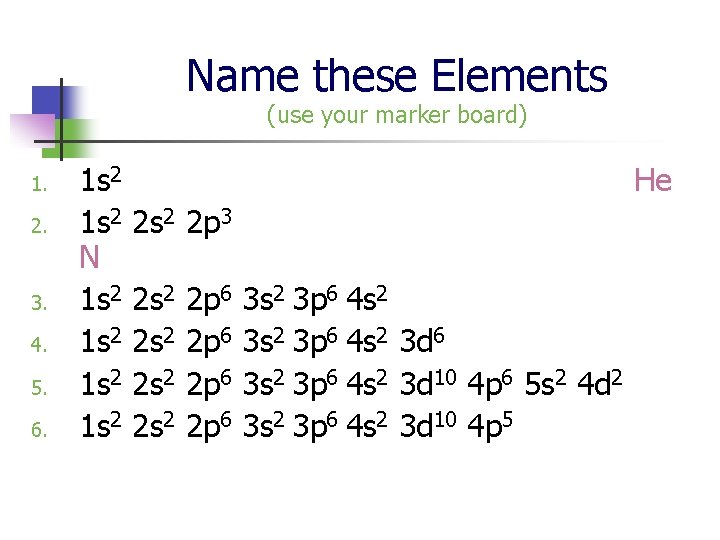
Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 2 s 2 2 p 3 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 He

Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 N 1 s 2 He 2 s 2 2 p 3 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5

Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 2 s 2 2 p 3 N 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 Ca 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 He

Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 N 1 s 2 Ca 1 s 2 Fe 1 s 2 He 2 s 2 2 p 3 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5

Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 N 1 s 2 Ca 1 s 2 Fe 1 s 2 He 2 s 2 2 p 3 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 Zr 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5

Name these Elements (use your marker board) 1. 2. 3. 4. 5. 6. 1 s 2 N 1 s 2 Ca 1 s 2 Fe 1 s 2 He 2 s 2 2 p 3 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 2 Zr 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 Br