Day 26 What do liquid water and water














- Slides: 16

Day 26 What do liquid water and water vapor have in common? They’re both made up of water particles that move randomly from place to place. How are liquid water and water vapor different? Water vapor particles move faster, bounce harder, and spread out more than liquid water particles.

Compare Evaporation and Condensation Contrasting Case Activity 6 Weather & Water, Investigation 6: Water in the Air

A Forgotten Towel Damien was swimming on a hot summer day. When he got out of the pool, he saw he had forgotten his towel. Even though it was a hot day, he started to feel cold. He sat in the sun and, a half hour later, he was dry and he felt warm again. How did Damien get dry without a towel?

Spilled Water Tameka was carrying a glass of water from the kitchen to her bedroom. As she was walking, she spilled some water on the floor. She forgot about it while she did her homework. When she went back an hour later, the floor was dry. How did the floor get dry?

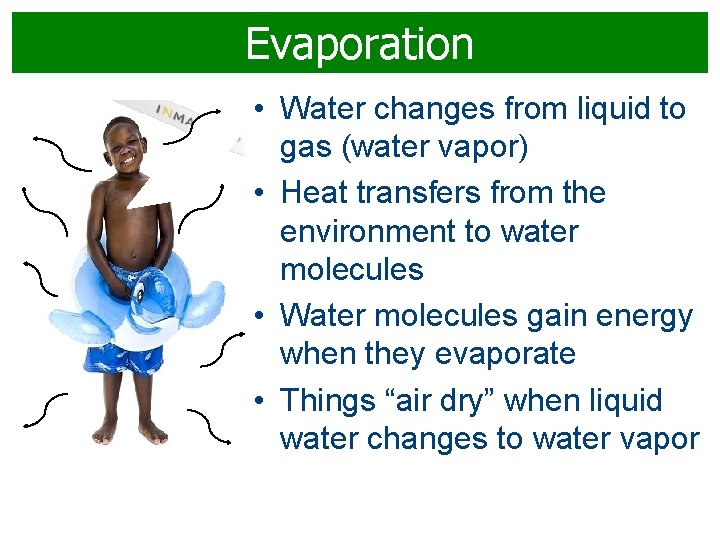
Evaporation • Water changes from liquid to gas (water vapor) • Heat transfers from the environment to water molecules • Water molecules gain energy when they evaporate • Things “air dry” when liquid water changes to water vapor

Answer Questions When Damien got out of the pool, he saw he had forgotten his towel. Even though it was a hot day, he started to feel cold. He sat in the sun and, a half hour later, he was dry and he felt warm again. As Tameka was carrying a glass of water to her bedroom, she spilled some on the floor. When she went back an hour later, the floor was dry.

Question 1 How did Damien get dry? The water on his skin evaporated. He felt cold because heat transferred from his skin to the liquid water molecules. When the liquid molecules gained energy, they changed to water vapor and joined the surrounding air.

Question 2 How did the floor get dry? The water on the floor evaporated. Heat transferred from the floor and surrounding air to the liquid water molecules. When the liquid molecules gained energy, they changed to water vapor and joined the surrounding air.

The Water Stain Raymond’s grandmother always scolded him for not using coasters to protect the table from water stains. One humid day, he poured a cold glass of soda and wiped the glass with a towel. The glass was dry when he set it down. Ten minutes later, the glass was wet and there was a water stain on the table. How did the table get wet?

Jasmine Sees her Breath Jasmine stepped outside one cold winter morning. As she walked, she noticed little clouds forming when she breathed. She wondered why she could only see her breath on cold mornings and not in summer, when the air is warm. Why did Jasmine see her breath?

Condensation • • moist air • • Water vapor in the air changes from gas to liquid Heat transfers from water molecules to the environment Water molecules lose energy when they condense Glasses "sweat" when water vapor changes to liquid water

Answer Questions Raymond poured himself a cold glass of soda. The glass was dry when he set it down. Ten minutes later, the glass was wet and there was a water stain on the table. One cold winter morning, Jasmine noticed little clouds forming when she breathed. She wondered why she could only see her breath on cold mornings and not when the air is warm.

Question 4 How did the table get wet? Water vapor in the surrounding air condensed. Heat transferred from the water vapor molecules to the cold glass. When the water vapor molecules lost energy, they changed to liquid water molecules that collected on the glass and dripped down onto the table.

Question 5 Why did Jasmine see her breath? As she exhaled, water vapor in the air from her lungs condensed. Heat transferred from the water vapor molecules to the cold air around her. When the water vapor molecules lost energy, they changed to liquid water molecules that collected in droplets to form a visible cloud.

Evaporation vs. Condensation Use your Evaporation and Condensation cards to compare and contrast the two processes. Warm air

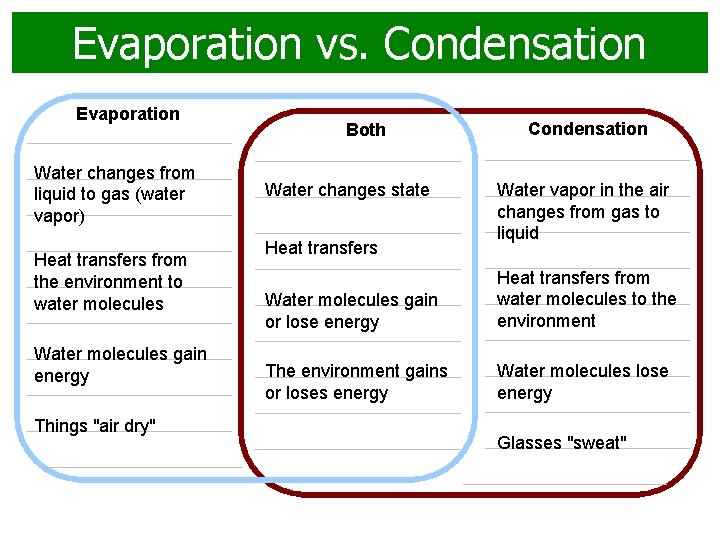
Evaporation vs. Condensation Evaporation Water changes from liquid to gas (water vapor) Heat transfers from the environment to water molecules Water molecules gain energy Things "air dry" Both Water changes state Heat transfers Condensation Water vapor in the air changes from gas to liquid Water molecules gain or lose energy Heat transfers from water molecules to the environment The environment gains or loses energy Water molecules lose energy Glasses "sweat"