Day 24 Chemistry 4 How do elements combine
Day 24 Chemistry 4. How do elements combine together to produce compounds that account for all living and non-living substances? 5. How can we describe and measure quantities related to chemical/physical changes within a system? 3. What evidence can we identify that supports the law of conservation of matter. ENGAGE: IT 10 min. Which element is. . . ? Use page 614 and 615 to fine which element matches the clue! 1 Half of a dime 2 The Lone Ranger's horse 3 A police officer 4 What I do when I'm hungry 5 What torpedoed ships do 6 Male member of the Ganese tribe 7 What he did with a bucking horse 8 What should be done with an ailing man 9 What to do with the dead 10 Frivolous prisoner

Atomic Structure Unit II—Part 3

What is an atom? • Atom: the smallest unit of matter that retains the identity of the substance • First proposed by Democratus
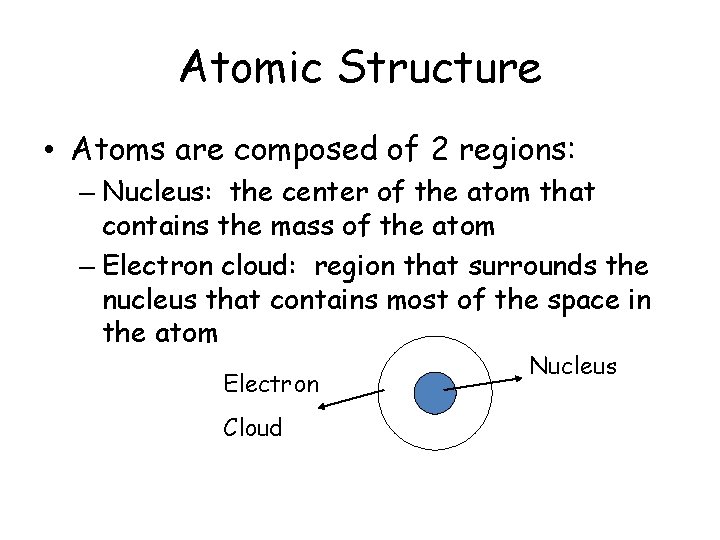
Atomic Structure • Atoms are composed of 2 regions: – Nucleus: the center of the atom that contains the mass of the atom – Electron cloud: region that surrounds the nucleus that contains most of the space in the atom Electron Cloud Nucleus

What’s in the Nucleus? • The nucleus contains 2 of the 3 subatomic particles: – Protons: positively charged subatomic particles – Neutrons: neutrally charged subatomic particles

What’s in the Electron Cloud? • The 3 rd subatomic particle resides outside of the nucleus in the electron cloud – Electron: the subatomic particle with a negative charge and relatively no mass

How do these particles interact? • Protons and neutrons live compacted in the tiny positively charged nucleus accounting for most of the mass of the atom • The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus

How do the subatomic particles balance each other? • In an atom: – The protons = the electrons • If 20 protons are present in an atom then 20 electrons are there to balance the overall charge of the atom—atoms are neutral – The neutrons have no charge; therefore they do not have to equal the number of protons or electrons
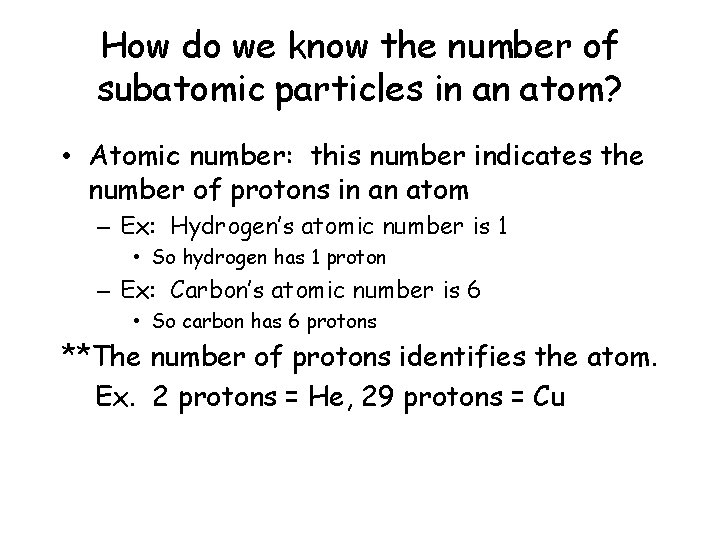
How do we know the number of subatomic particles in an atom? • Atomic number: this number indicates the number of protons in an atom – Ex: Hydrogen’s atomic number is 1 • So hydrogen has 1 proton – Ex: Carbon’s atomic number is 6 • So carbon has 6 protons **The number of protons identifies the atom. Ex. 2 protons = He, 29 protons = Cu

How do we know the number of subatomic particles in an atom? • Mass number: the number of protons and neutrons in the nucleus – Ex: hydrogen can have a mass of 3. Since it has 1 proton it must have 2 neutrons – # of neutrons = mass # - atomic #

Determining the number of protons and neutrons • Li has a mass number of 7 and an atomic number of 3 – Protons = 3 (same as atomic #) – Neutrons= 7 -3 = 4 (mass # - atomic #) • Ne has a mass number of 20 and an atomic number of 10 – Protons = 10 – Neutrons = 20 - 10= 10
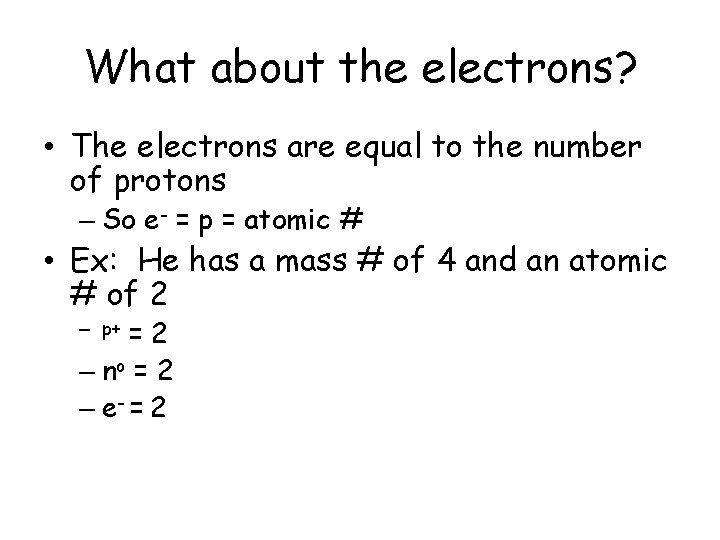
What about the electrons? • The electrons are equal to the number of protons – So e- = p = atomic # • Ex: He has a mass # of 4 and an atomic # of 2 =2 – no = 2 – e- = 2 – p+
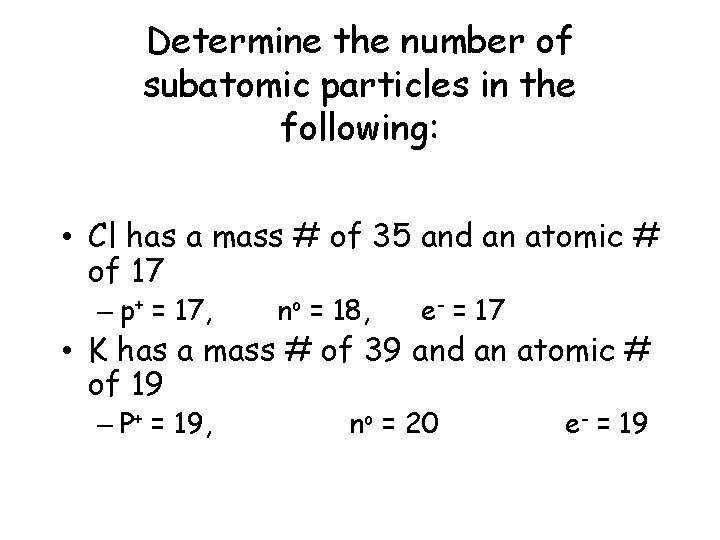
Determine the number of subatomic particles in the following: • Cl has a mass # of 35 and an atomic # of 17 – p+ = 17, no = 18, e- = 17 • K has a mass # of 39 and an atomic # of 19 – P+ = 19, no = 20 e- = 19
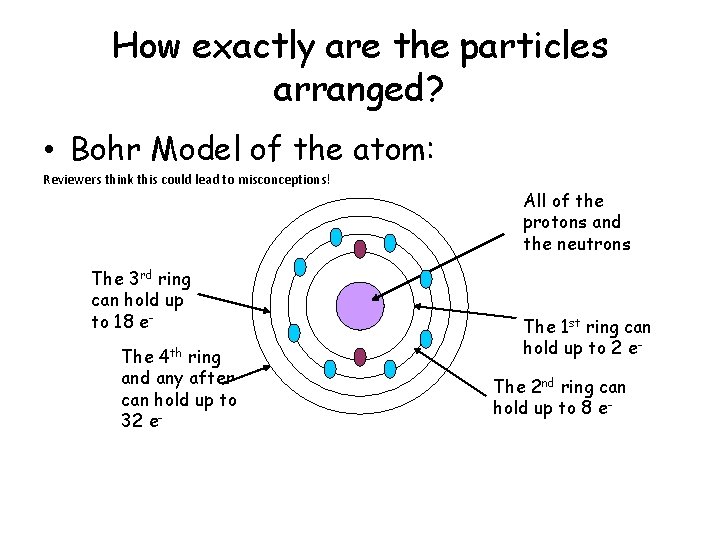
How exactly are the particles arranged? • Bohr Model of the atom: Reviewers think this could lead to misconceptions! The 3 rd ring can hold up to 18 e. The 4 th ring and any after can hold up to 32 e- All of the protons and the neutrons The 1 st ring can hold up to 2 e. The 2 nd ring can hold up to 8 e-
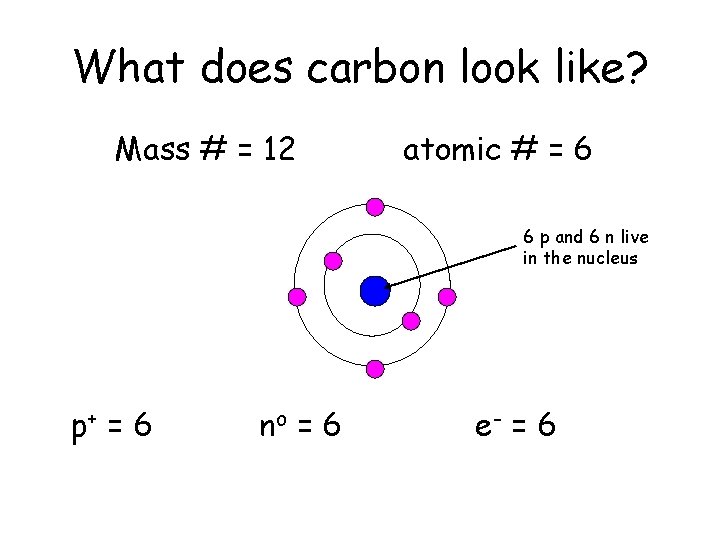
What does carbon look like? Mass # = 12 atomic # = 6 6 p and 6 n live in the nucleus p+ = 6 no = 6 e- = 6

Independent Assignments 1. Complete your notes using the power point on canvas. Turn this in with your NAME ON the paper! 2. Complete the Lewis Structure and Bohr diagram practice sheet. Turn this in with your NAME ON the paper! 3. Work on your Superhero/Villain project independently. 4. Work on your vocabulary (see next slide for details)

Elements and the Periodic Table: pages 604 -635 and any notes provided Nucleus Proton Neutron Electron Atomic number Atomic mass Model Periodic table Period (Periodic Table) Group (Periodic Table) Metal (periodic Table Nonmetal (periodic Table) Metalloid (periodic table) Bohr Model Lewis Structure Balancing Equations Chemicals and the effects on humans Vocabulary due Monday April 24 th May Test Tuesday 9 th May For your vocabulary, complete either #1 or #2. You need to know and understand each concept. 1. ) Give definitions and examples and diagrams in your notebook/cards/power point 2. ) Create a mind map connecting the information on the study guide

Extra Chemistry Practice – http: //chemsite. lsrhs. net/bonding/flash. Lewis. html Lewis structure practice – http: //www. zerobio. com/drag_gr 9/bohr. htm Bohr Diagram practice – https: //www. quia. com/quiz/303980. html – http: //studyjams. scholastic. com/studyjams/science/ matter/changes-of-matter. htm – – – - Atoms Elements Compounds - Matter http: //www. flashcardmachine. com/3141520/4 g 3 q Periodic Table - Matter http: //www. flashcardmachine. com/3244178/j 69 w Physical/Chemical Properties - Matter http: //www. flashcardmachine. com/3244183/4 y 7 o Law of Conservation of Mass - Matter http: //www. flashcardmachine. com/3244189/t 15 z Physical or Chemical Change? https: //www. quia. com/quiz/303980. html Atom and Element Game https: //www. quia. com/rr/143370. html Chemical Equations CHALLENGE!!!! https: //www. quia. com/rr/85795. html
- Slides: 18