Day 23 Class Notes Review Balancing Chemical Equations
Day 2/3 Class Notes
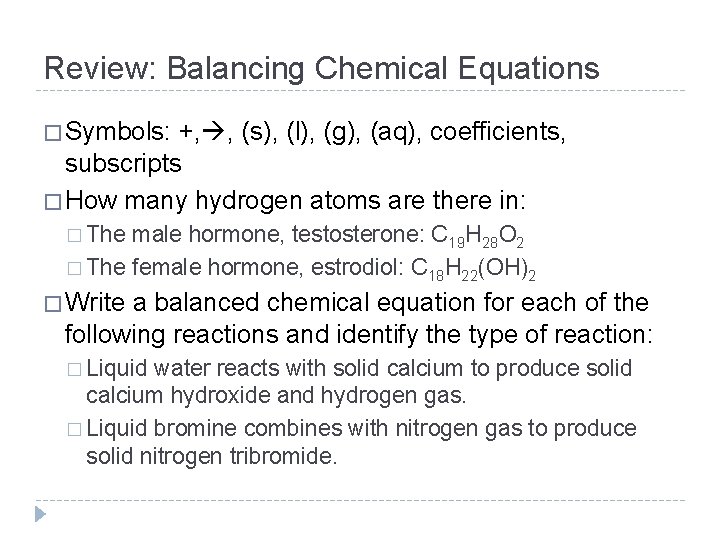
Review: Balancing Chemical Equations � Symbols: +, , (s), (l), (g), (aq), coefficients, subscripts � How many hydrogen atoms are there in: � The male hormone, testosterone: C 19 H 28 O 2 � The female hormone, estrodiol: C 18 H 22(OH)2 � Write a balanced chemical equation for each of the following reactions and identify the type of reaction: � Liquid water reacts with solid calcium to produce solid calcium hydroxide and hydrogen gas. � Liquid bromine combines with nitrogen gas to produce solid nitrogen tribromide.
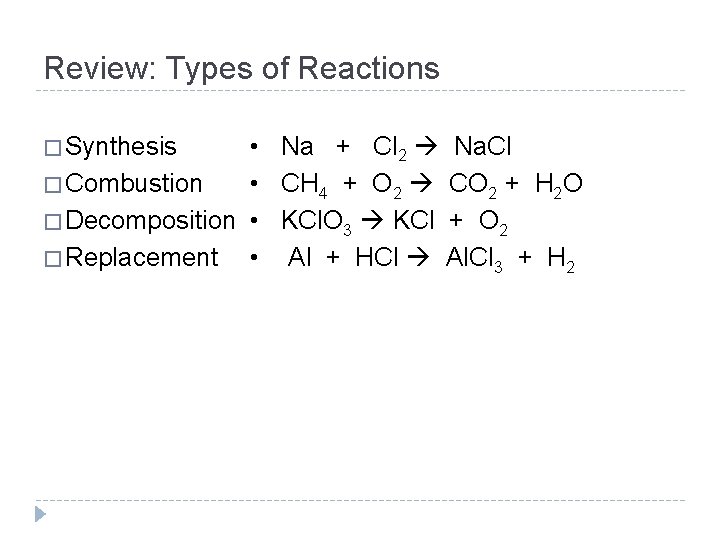
Review: Types of Reactions • Na + Cl 2 Na. Cl • CH 4 + O 2 CO 2 + H 2 O � Combustion � Decomposition • KCl. O 3 KCl + O 2 • Al + HCl Al. Cl 3 + H 2 � Replacement � Synthesis
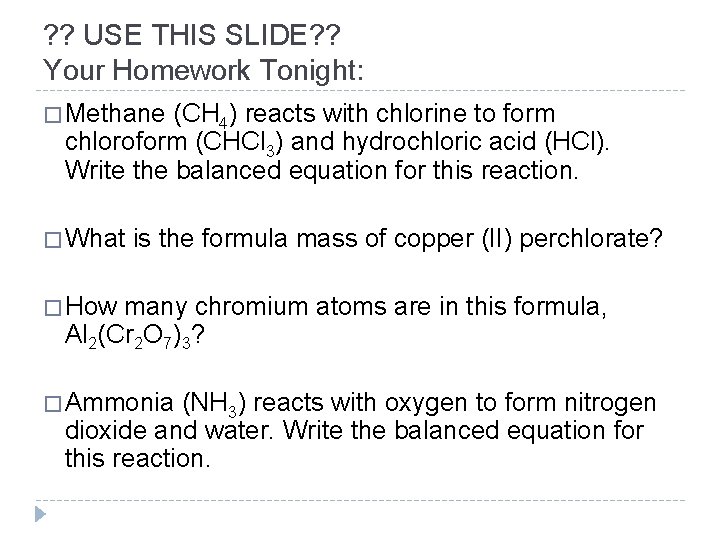
? ? USE THIS SLIDE? ? Your Homework Tonight: � Methane (CH 4) reacts with chlorine to form chloroform (CHCl 3) and hydrochloric acid (HCl). Write the balanced equation for this reaction. � What is the formula mass of copper (II) perchlorate? � How many chromium atoms are in this formula, Al 2(Cr 2 O 7)3? � Ammonia (NH 3) reacts with oxygen to form nitrogen dioxide and water. Write the balanced equation for this reaction.

Atomic Mass/Formula Mass/Molar Mass � What is the mass of C? 12. 011 amu � What is the mass of H? 1. 008 amu � What is the mass of O? 15. 999 amu � What happens if we have a compound? What is the mass of H 2 O? 1. 008(2) + 15. 999 = 18. 01 amu 18. 01 g/mol
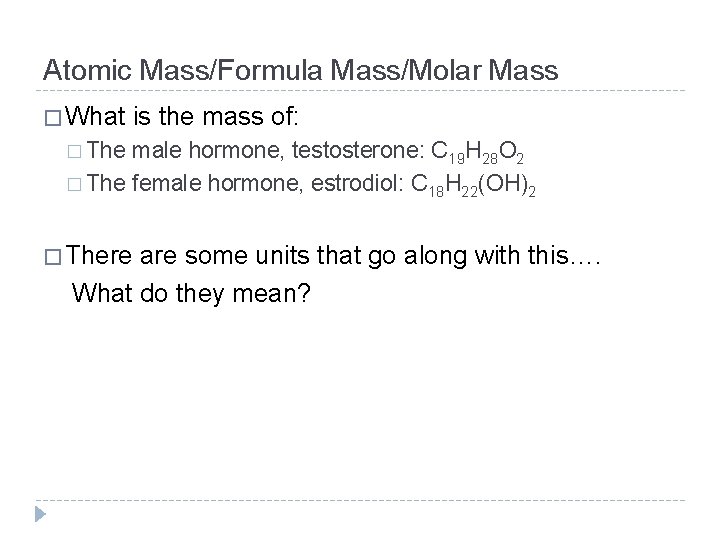
Atomic Mass/Formula Mass/Molar Mass � What is the mass of: � The male hormone, testosterone: C 19 H 28 O 2 � The female hormone, estrodiol: C 18 H 22(OH)2 � There are some units that go along with this…. What do they mean?

The mole, Avogadro’s Number, and converting between them.

How many are in the jar?

How many are in the jar?

Counting: � PAIR: � DOZEN: � REAM: � MOLE: � aka: Avogadro’s number 6. 022*1023

� http: //ed. ted. com/lessons/daniel-dulek-how-big-is-a- mole-not-the-animal-the-other-one

The MOLE and Avogadro’s Number The following analogies will help you to understand the enormous, almost incomprehensible, size of Avogadro's Number (6. 022 X 1023). � It stands for six-hundred-two-sextillion-two-hundred-quintillion 602, 213, 670, 000, 000 � Avogadro's Number of water drops would fill a swimming pool of circular dimensions with a diameter of 1 mile from the earth to the sun (a distance of 93 million miles). � There approximately two times Avogadro's Number of milliliters of water on earth. � For a total of Avogadro's Number of frames of film to have been viewed, each person on Earth (7 billion) would have to sit through 1. 0 billion showings of "Return of the Jedi".

� To store Avogadro's Number of bits of information using advanced 4 megabit (million bit) chips would require about a cubic mile of chips. � If you divide Avogadro's Number of pennies equally among every man, woman, and child in the United States, each person could pay off the national debt (currently $1. 7 trillion) and still have 20 trillion dollars or so left for incidental expenses. � If you had a fortune worth Avogadro's Number of dollars, you could spend a billion dollars each second of your entire life and have used only about. 001% of your money. � Avogadro's Number of dollars could not be spent at the rate of a billion dollars a day over one trillion years.

� Avogadro's Number of marbles, spread over the surface of the Earth, would produce a layer of marbles about 50 miles thick. � If carbon atoms were the size of peas, Avogadro's Number of them would cover the surface of the earth to a depth of 15 meter easily burying the Jolly Green Giant. � If you have Avogadro's Number of unpopped popcorn kernels, and pour them over the continental United States, the country would be covered to a depth of 9 miles. � Avogadro's Number of grains of sand, spread over the United States, would produce a layer of sand about 3 inches deep.

� What is used as the basis for Avogadro’s number (a mole)? � the number of carbon atoms in exactly 12 g of pure carbon-12. � A mole ALWAYS contains the SAME number of particles; however, moles of DIFFERENT substances have DIFFERENT masses. � Just like if you bought a dozen cookies, or a dozen M&Ms, or a dozen apples, you have TWELVE of EACH, YET they do NOT WEIGH the same.

� Molar Mass � the mass in grams of one mole of any pure substance � (Look to the periodic table!) � Formula Mass � Atomic Mass
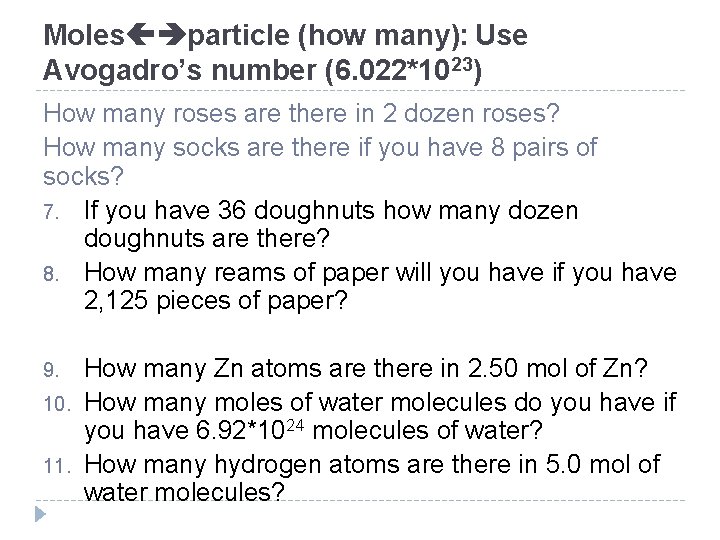
Moles particle (how many): Use Avogadro’s number (6. 022*1023) How many roses are there in 2 dozen roses? How many socks are there if you have 8 pairs of socks? 7. If you have 36 doughnuts how many dozen doughnuts are there? 8. How many reams of paper will you have if you have 2, 125 pieces of paper? 9. 10. 11. How many Zn atoms are there in 2. 50 mol of Zn? How many moles of water molecules do you have if you have 6. 92*1024 molecules of water? How many hydrogen atoms are there in 5. 0 mol of water molecules?
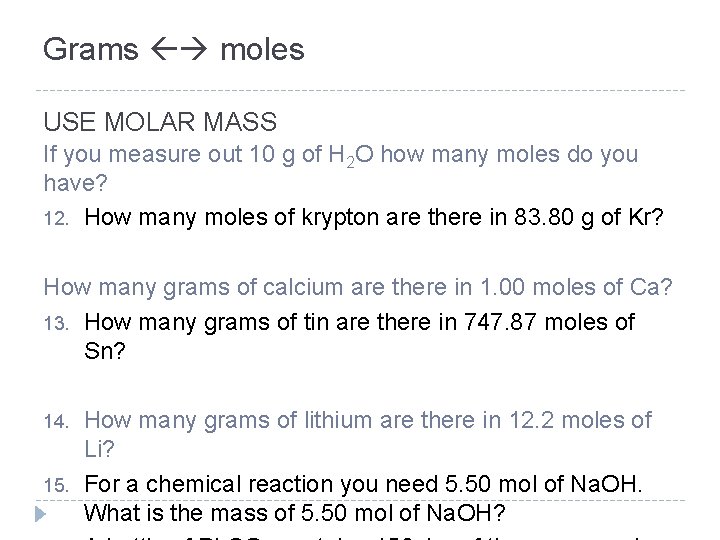
Grams moles USE MOLAR MASS If you measure out 10 g of H 2 O how many moles do you have? 12. How many moles of krypton are there in 83. 80 g of Kr? How many grams of calcium are there in 1. 00 moles of Ca? 13. How many grams of tin are there in 747. 87 moles of Sn? 14. 15. How many grams of lithium are there in 12. 2 moles of Li? For a chemical reaction you need 5. 50 mol of Na. OH. What is the mass of 5. 50 mol of Na. OH?

Particles (how many) moles USE AVOGADRO’S NUMBER 17. 18. 19. 20. Determine the number of atoms in 0. 58 moles of Se. How many molecules of nitroglycerine are in 12. 2 mol of nitroglycerine? How many moles of barium nitrate are in 6. 80 *1024 molecules of barium nitrate? How many moles of caffeine are in 9. 37*1042 molecules of caffeine?

Two-step problem: � You need 56. 3 g of copper to make a copper bracelet. How many atoms of copper will be in the bracelet?
- Slides: 20