Day 1 Recognizing and Balancing Redox Reactions Redox


Day 1: Recognizing and Balancing Redox Reactions

Redox Reactions: What are they? Any chemical reaction where electrons are transferred between reactants For example: Mg + O 2 Mg. O Although it can’t be directly observed, as this rxn happens, Mg atoms transfer electrons to O atoms

Redox Reactions: How do we ID them? If the charges (oxidation numbers) of the atoms change between reactants and products, then there was probably an electron transfer For example: Mg + O 2 Mg. O

Redox Reactions: How do we assign oxidation numbers? 1. All un-combined atoms have O. N. ’s = zero 2. Any monatomic ion has an O. N. = it’s charge 3. Oxygen has an O. N. = -2 except in peroxides when it is -1 4. Hydrogen has an O. N. = +1 except in metal hydrides when it is -1 5. The sum of all the O. N. ’s in a neutral compound must be zero, and in a polyatomic ion they must add up to the charge of the ion.

Redox Reactions: Practice Assign oxidation numbers to each element in the species given below Na 2 O 2 sodium peroxide Mn. O 4 -1 permanganate ion Mg(NO 3)2 magnesium nitrate

Redox Reactions: Writing Half Reactions It is useful to think about redox reactions as two separate reactions (half reactions) that happen simultaneously For example: Mg + O 2 Mg. O

Redox Reactions: Practice Na + Br 2 Na. Br Identify which element is oxidized, which is reduced, and write the oxidation and reduction half reactions.
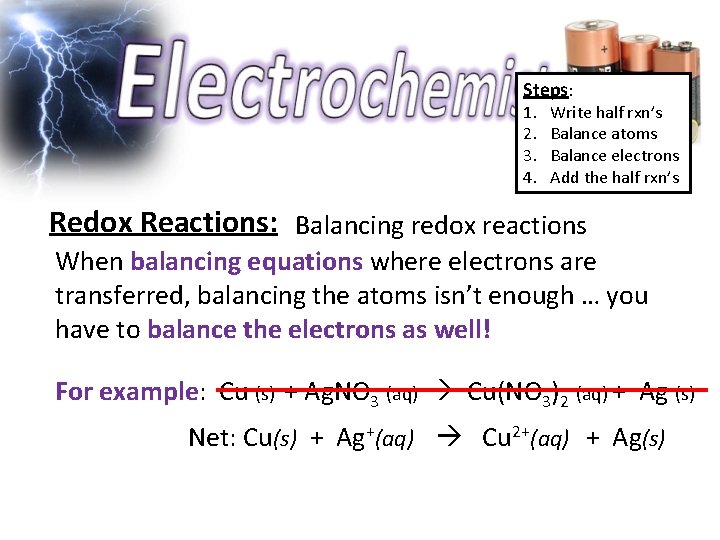
Steps: 1. 2. 3. 4. Write half rxn’s Balance atoms Balance electrons Add the half rxn’s Redox Reactions: Balancing redox reactions When balancing equations where electrons are transferred, balancing the atoms isn’t enough … you have to balance the electrons as well! For example: Cu (s) + Ag. NO 3 (aq) Cu(NO 3)2 (aq) + Ag (s) Net: Cu(s) + Ag+(aq) Cu 2+(aq) + Ag(s)
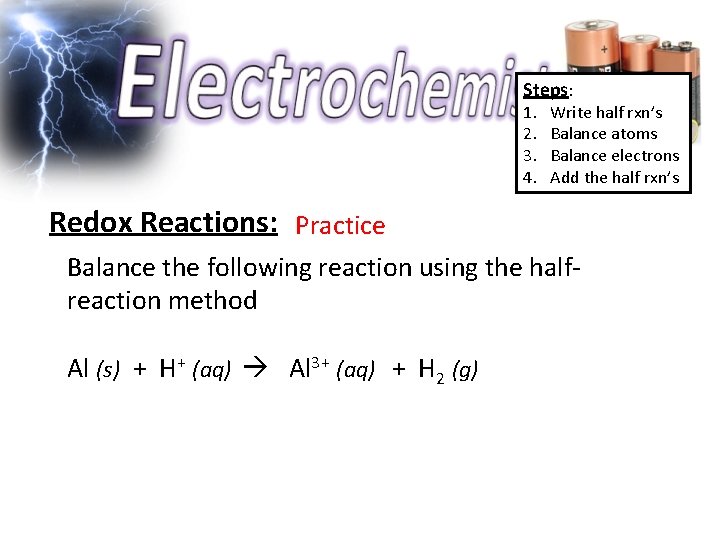
Steps: 1. 2. 3. 4. Write half rxn’s Balance atoms Balance electrons Add the half rxn’s Redox Reactions: Practice Balance the following reaction using the halfreaction method Al (s) + H+ (aq) Al 3+ (aq) + H 2 (g)

Steps: 1. 2. 3. 4. Write half rxn’s Balance atoms Balance electrons Add the half rxn’s Practice!!! In the packet, try #1, 2, 3 and 6 to practice ID’ing redox reactions and writing half reactions Then try #13, 14, 16 and 18 to try balancing using the half reaction method.
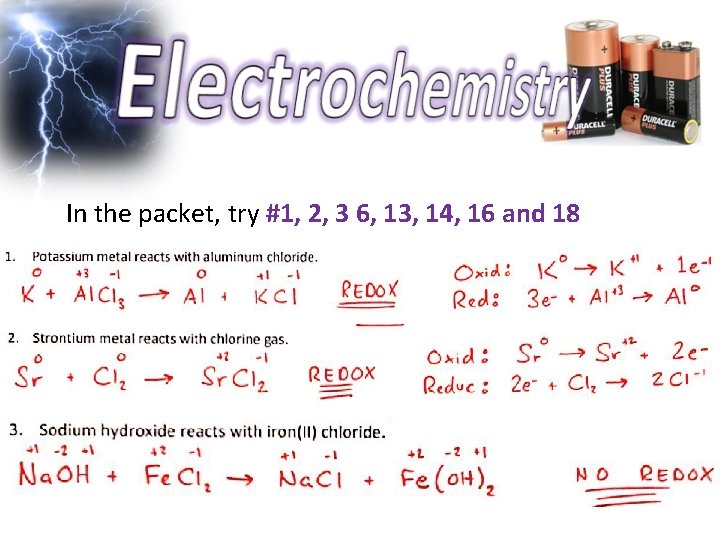
In the packet, try #1, 2, 3 6, 13, 14, 16 and 18
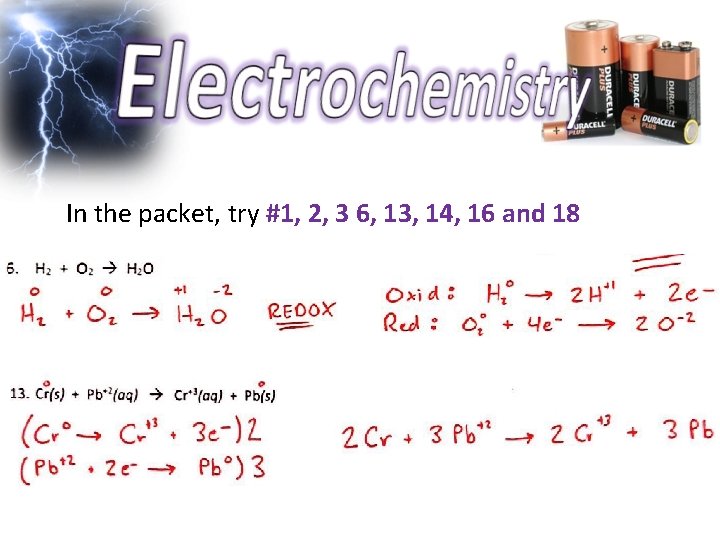
In the packet, try #1, 2, 3 6, 13, 14, 16 and 18
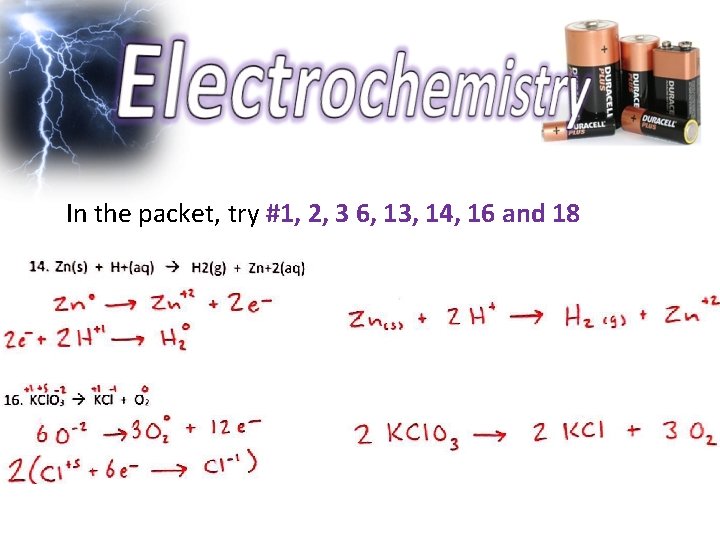
In the packet, try #1, 2, 3 6, 13, 14, 16 and 18
- Slides: 14