Dating the Earth using radioactive isotopes How do






- Slides: 12

Dating the Earth using radioactive isotopes

How do they know it is that old • The estimated age of the Earth is put at 4. 5 billion years old. • THAT’S OLD!!! If you had a dollar for every year, you could spend $4, 500 a day for 1, 904 years!! • Nobody was around that long ago, so how do they know it is that old?

Radiometric dating, what is it really?

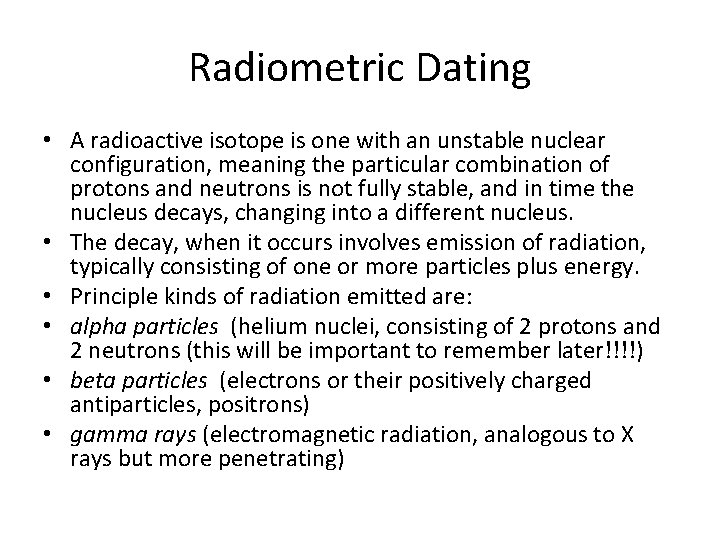
Radiometric Dating • A radioactive isotope is one with an unstable nuclear configuration, meaning the particular combination of protons and neutrons is not fully stable, and in time the nucleus decays, changing into a different nucleus. • The decay, when it occurs involves emission of radiation, typically consisting of one or more particles plus energy. • Principle kinds of radiation emitted are: • alpha particles (helium nuclei, consisting of 2 protons and 2 neutrons (this will be important to remember later!!!!) • beta particles (electrons or their positively charged antiparticles, positrons) • gamma rays (electromagnetic radiation, analogous to X rays but more penetrating)

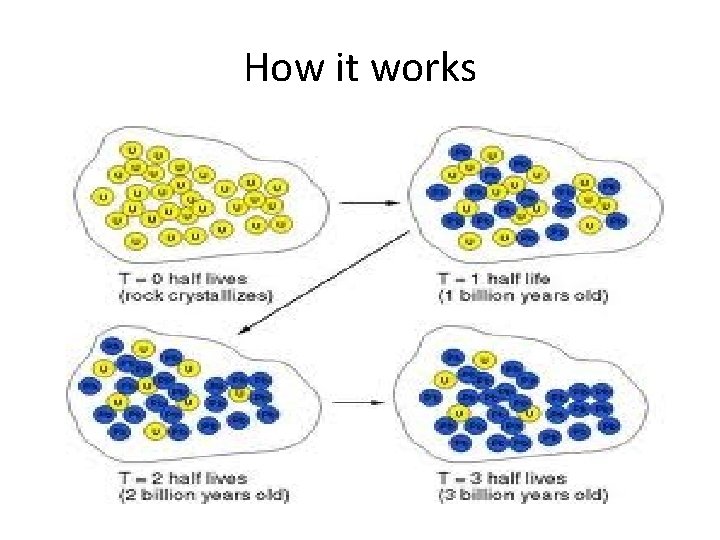
How it works

Parent and Daughter nuclei • The decaying nucleus by convection is called the parent nucleus • The nucleus into which it decays is called the daughter nucleus

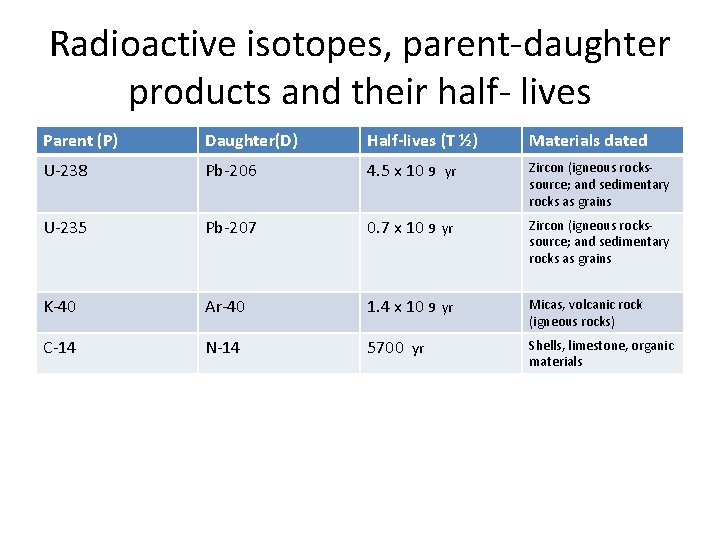
Radioactive isotopes, parent-daughter products and their half- lives Parent (P) Daughter(D) Half-lives (T ½) Materials dated U-238 Pb-206 4. 5 x 10 9 yr Zircon (igneous rockssource; and sedimentary rocks as grains U-235 Pb-207 0. 7 x 10 9 yr Zircon (igneous rockssource; and sedimentary rocks as grains K-40 Ar-40 1. 4 x 10 9 yr Micas, volcanic rock (igneous rocks) C-14 N-14 5700 yr Shells, limestone, organic materials

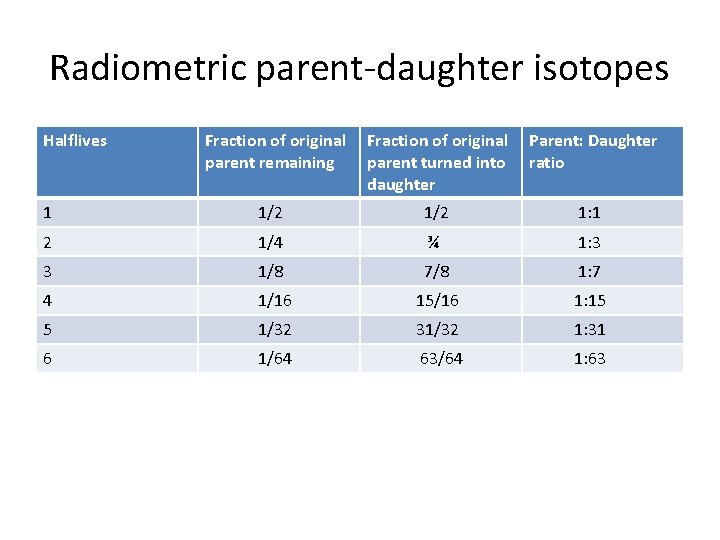
Radiometric parent-daughter isotopes Halflives Fraction of original parent remaining Fraction of original parent turned into daughter Parent: Daughter ratio 1 1/2 1: 1 2 1/4 ¾ 1: 3 3 1/8 7/8 1: 7 4 1/16 15/16 1: 15 5 1/32 31/32 1: 31 6 1/64 63/64 1: 63

Important things to remember Not all rocks can be dated radiometrically. 1. Some because they cannot maintain closed systems (like metamorphic rocks) 2. Others because they do not contain radioactive isotopes (like quartz sandstones) 3. Some because the radioactive isotopes that they do contain have half-lives that are either too long or too short to be measured for a rock of a certain age like trying to date a 1 m. y. -old rock by using C-14 decay- which would have been completely decayed after about 150, 000 years

Important issues to remember!! • Humans cannot affect the rate of decay of radioactive materials. • An unstable nucleus cannot be made more of less stable; we cannot speed up or slow down or stop the decay. • This makes the issue of the radioactive waste management a tremendous problem, radioactive wastes cannot be treated to make them nonradioactive thus they must decay naturally.

“Absolute” ages? • This term “Absolute” ages is actually incorrect. “Radiometric dating” means that something is given an actual age or date which is much different then relative dating which we covered earlier. • An example of absolute age would be counting the rings in a tree. • Dates calculated on the basis of decay of radioisotopes is radiometric dating, which is used to age or date rocks.
How old is the Earth really? • Page 321