Dating Techniques Four Categories Radioisotope methods Paleomagnetic methods















- Slides: 37

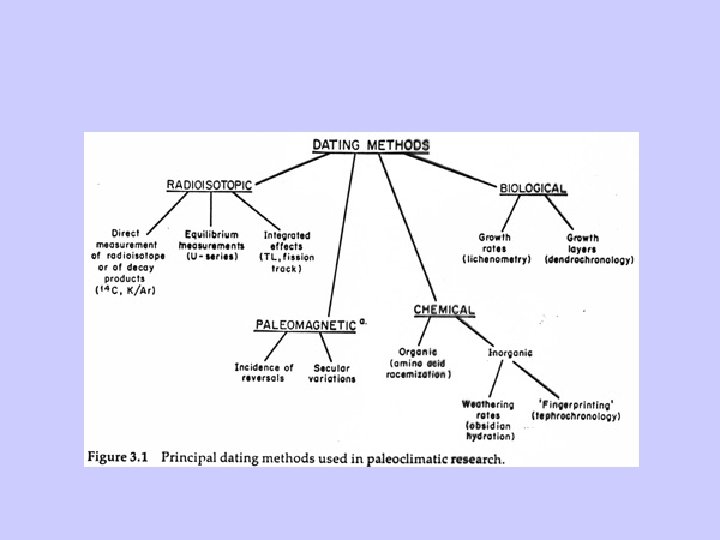
Dating Techniques • Four Categories – Radio-isotope methods – Paleomagnetic methods – Organic/inorganic chemical methods – Biological methods



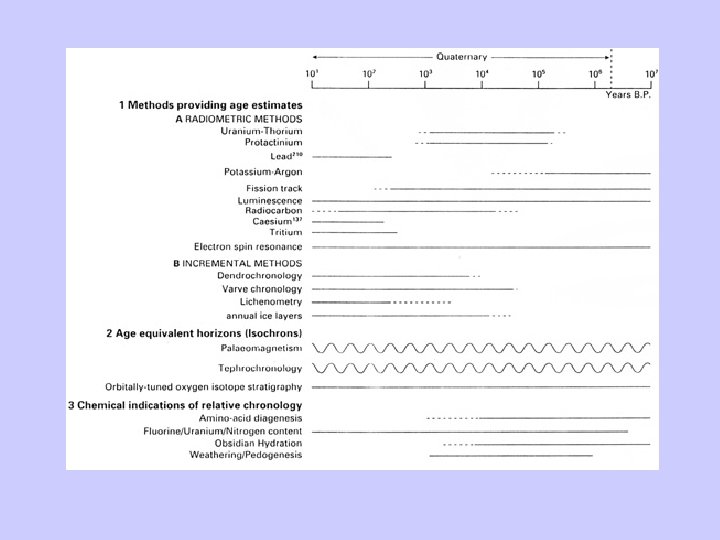
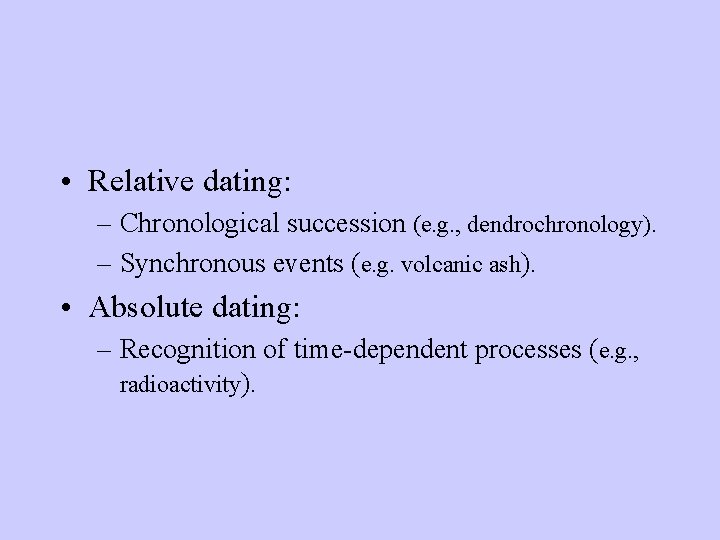
• Relative dating: – Chronological succession (e. g. , dendrochronology). – Synchronous events (e. g. volcanic ash). • Absolute dating: – Recognition of time-dependent processes (e. g. , radioactivity).

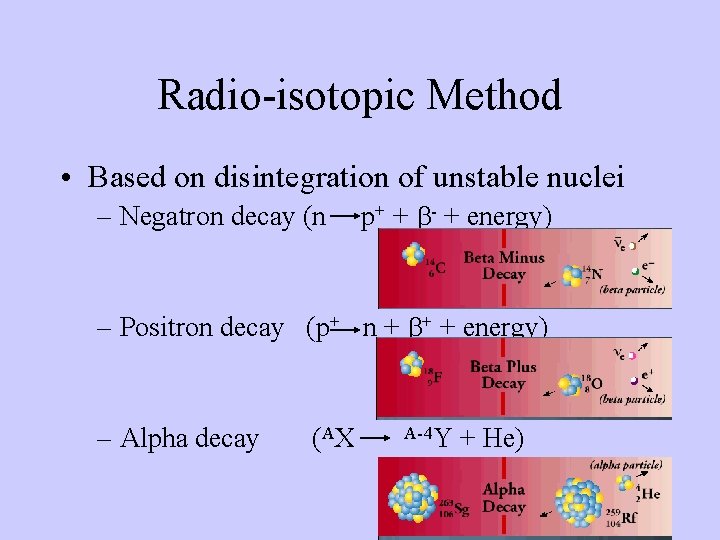
Radio-isotopic Method • Based on disintegration of unstable nuclei – Negatron decay (n p+ + b- + energy) – Positron decay (p+ n + b+ + energy) – Alpha decay (A X A-4 Y + He)

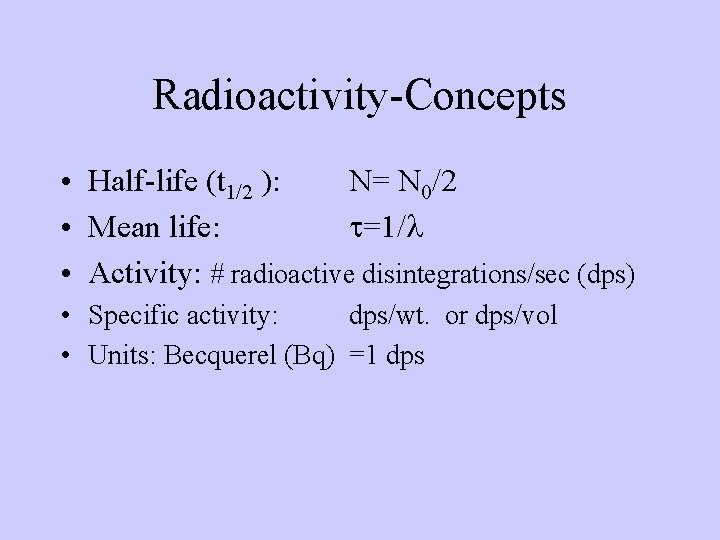
Radioactivity-Concepts • Half-life (t 1/2 ): N= N 0/2 • Mean life: t=1/l • Activity: # radioactive disintegrations/sec (dps) • Specific activity: dps/wt. or dps/vol • Units: Becquerel (Bq) =1 dps

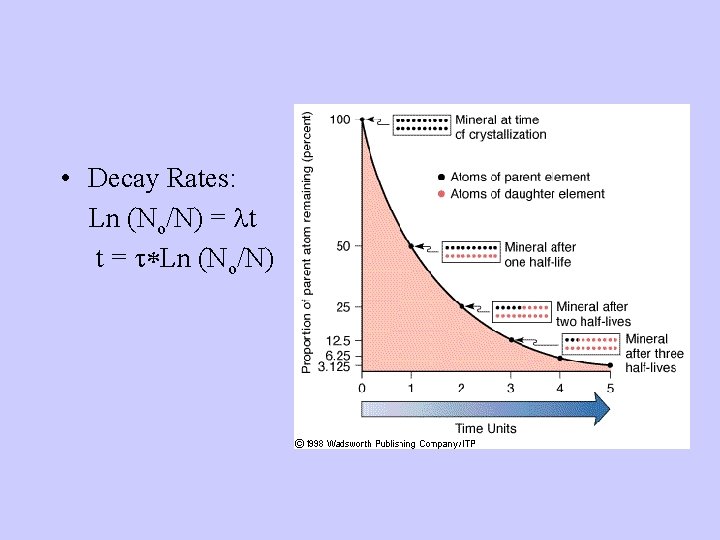
• Decay Rates: Ln (No/N) = lt t = t*Ln (No/N)

To be a useful for dating, radioisotopes must: • • • be measurable have known rate of decay have appropriate t 1/2 have known initial concentrations be a connection between event and radioisotope

Radioactivity-based Dating • Quantity of the radio-isotope relative to its initial level (e. g. , 14 C). • Equilibrium /non-equilibrium chain of radioactive decay (e. g. , U-series). • Physical changes on sample materials caused by local radioactive process (e. g. , fission track).

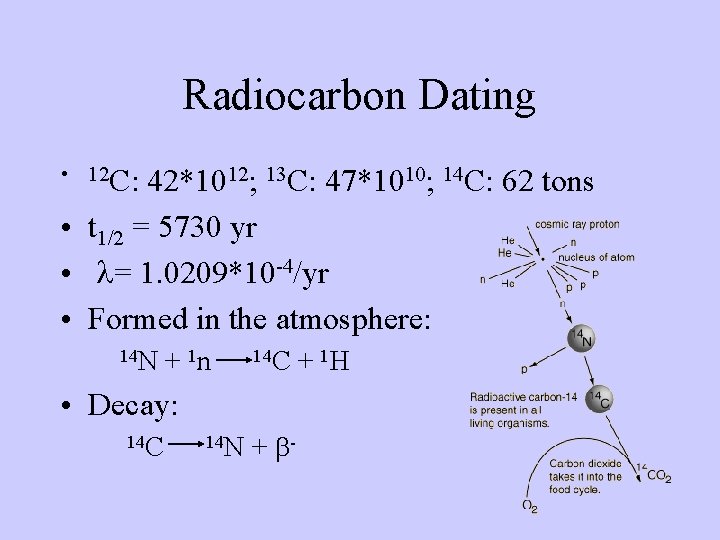
Radiocarbon Dating • 12 C: 42*1012; 13 C: 47*1010; 14 C: 62 tons • t 1/2 = 5730 yr • l= 1. 0209*10 -4/yr • Formed in the atmosphere: 14 N + 1 n 14 C • Decay: 14 C 14 N + b- + 1 H


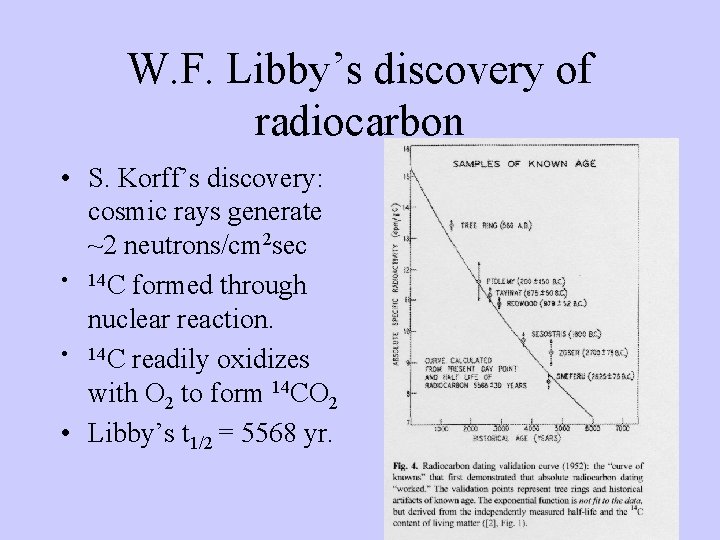
W. F. Libby’s discovery of radiocarbon • S. Korff’s discovery: cosmic rays generate ~2 neutrons/cm 2 sec • 14 C formed through nuclear reaction. • 14 C readily oxidizes with O 2 to form 14 CO 2 • Libby’s t 1/2 = 5568 yr.

Conventional Radiocarbon Dating • • Current t 1/2 = 5730± 40 yr t=8033*Ln(Asample/Astandard), where A: activity. Oxalic acid is the standard (prepared in 1950). Dates reported back in time relative to 1950 (radiocarbon yr BP). • Astandard in 1950 = 0. 227 Bq/g • Astandard in 2000 = 0. 225 Bq/g

Conventional Radiocarbon dating • Activity of 14 C needs to be “normalized” to the abundance of carbon: • D 14 C: “normalized value” » D 14 C(‰) = d 14 C – 2(d 13 C+25)(1+d 13 C/103) » d 14 C(‰) = (1 -Asample/Astandard)*103 • Radiocarbon age = 8033*ln(1+ D 14 C/103)

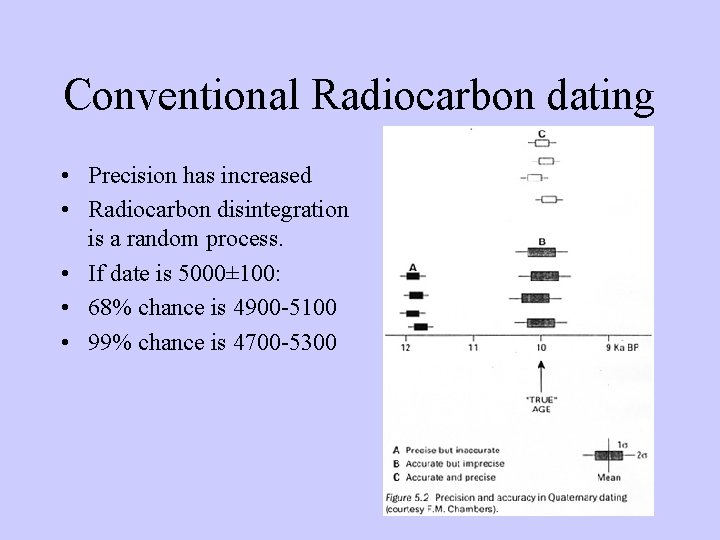
Conventional Radiocarbon dating • Precision has increased • Radiocarbon disintegration is a random process. • If date is 5000± 100: • 68% chance is 4900 -5100 • 99% chance is 4700 -5300


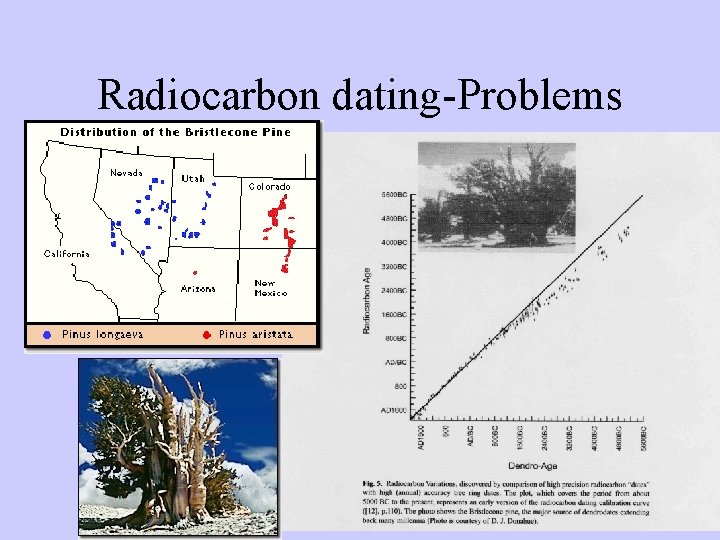
Radiocarbon dating-Problems

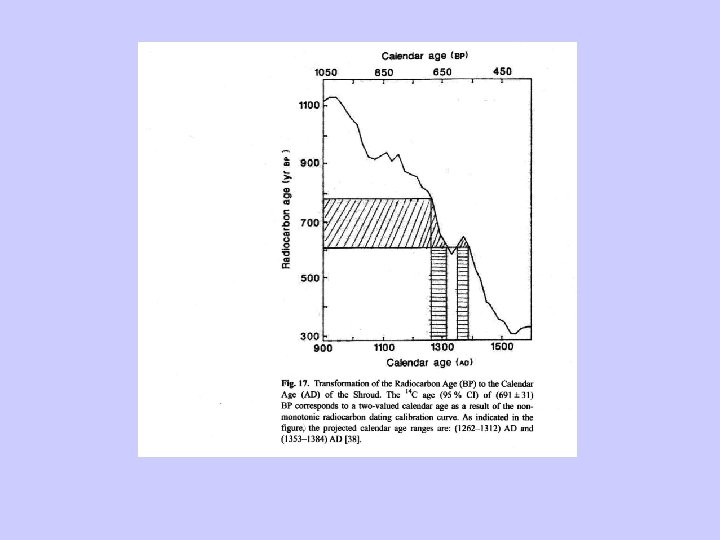
Radiocarbon dating-Corrections • Radiocarbon can be corrected by using tree -ring chronology. • Radiocarbon dates can then be converted into “Calendar years” (cal yr).



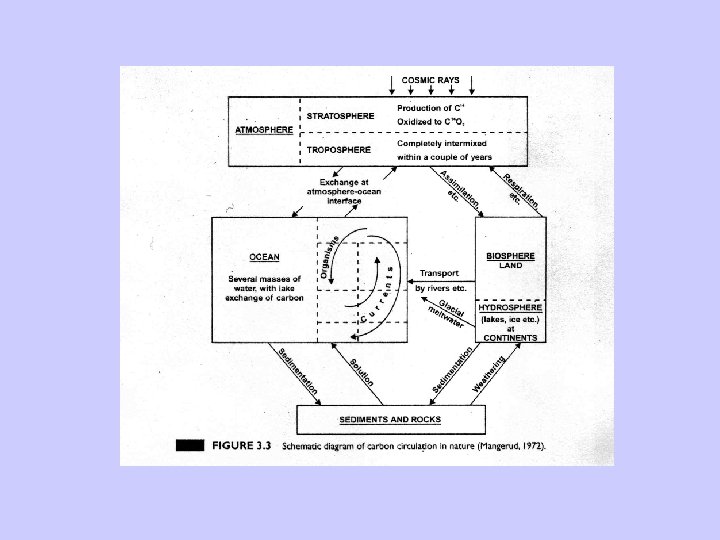
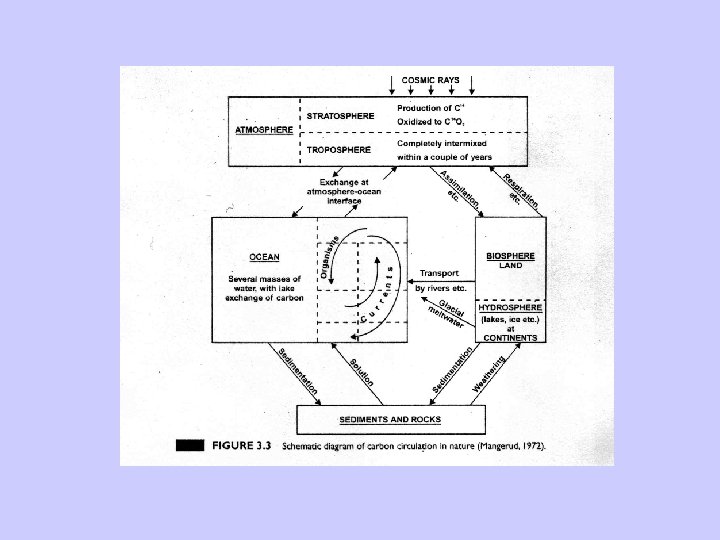
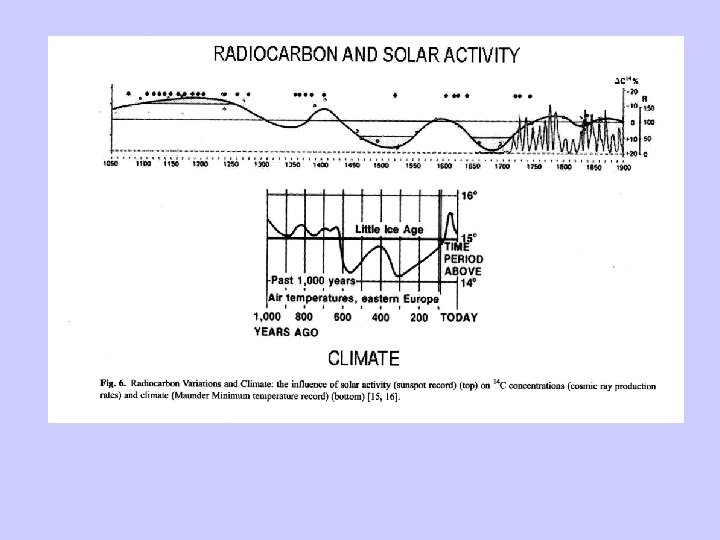
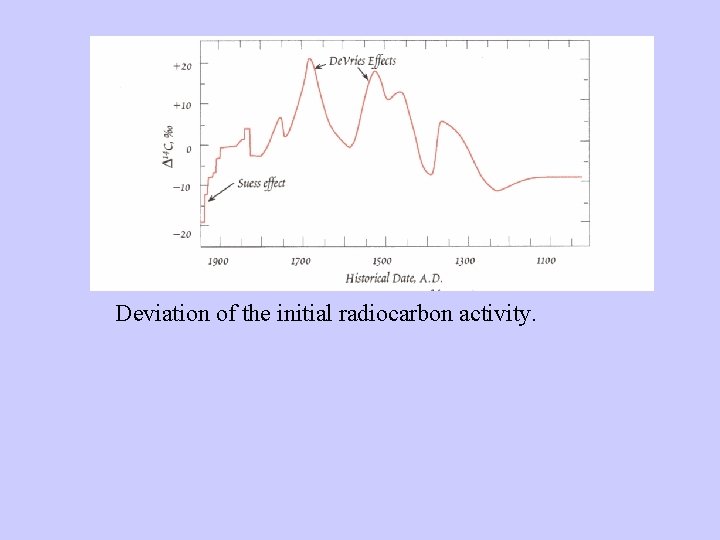
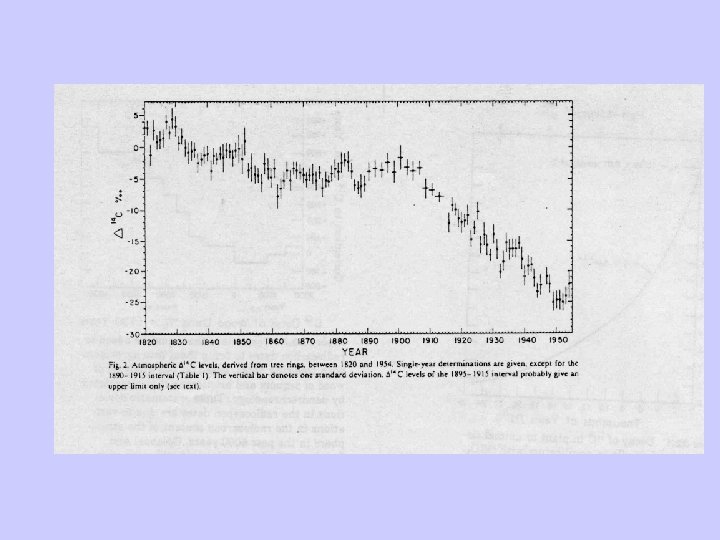
Radiocarbon dating-Problems • Two assumptions: – Constant cosmic ray intensity. – Constant size of exchangeable carbon reservoir. • Deviation relative to dendrochronology due to: – Variable 14 C production rates. – Changes in the radiocarbon reservoirs and rates of carbon transfer between them. – Changes in total amount of CO 2 in atmosphere, hydrosphere, and atmosphere.



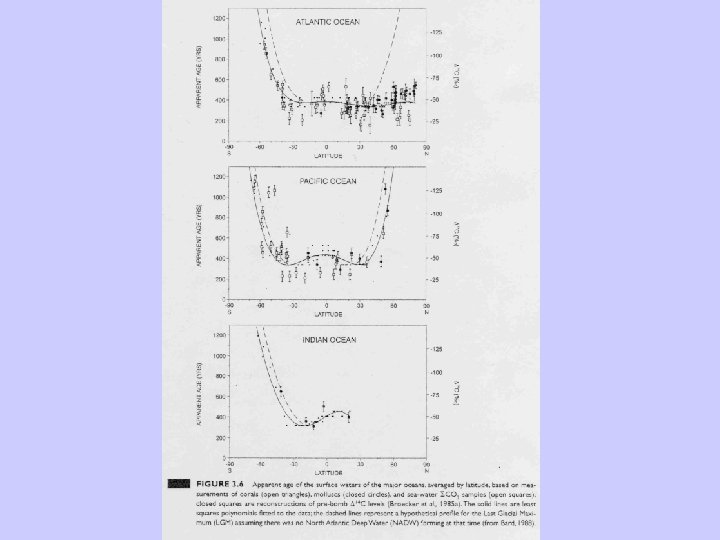
Deviation of the initial radiocarbon activity.


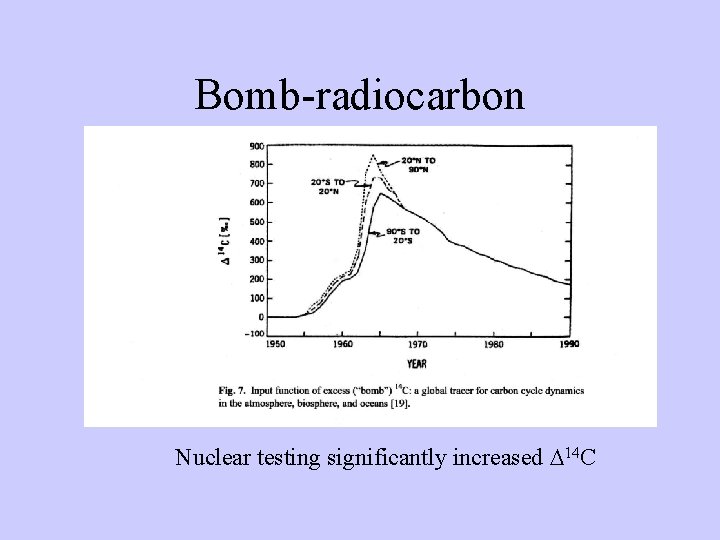
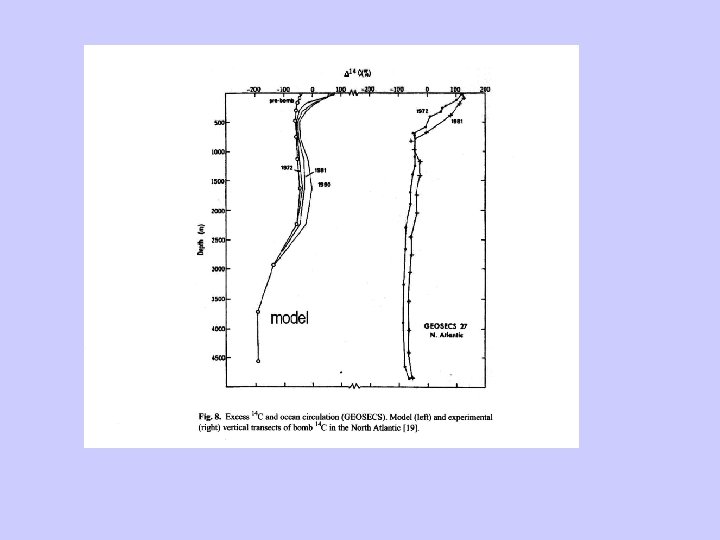
Bomb-radiocarbon Nuclear testing significantly increased D 14 C

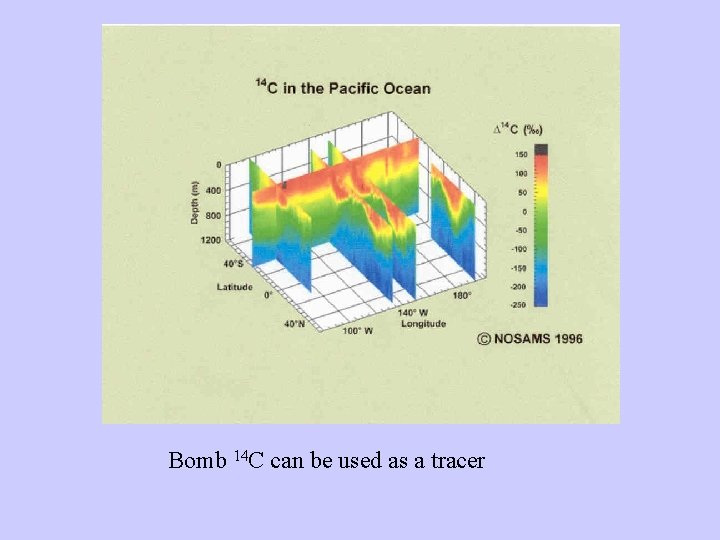
Bomb 14 C can be used as a tracer



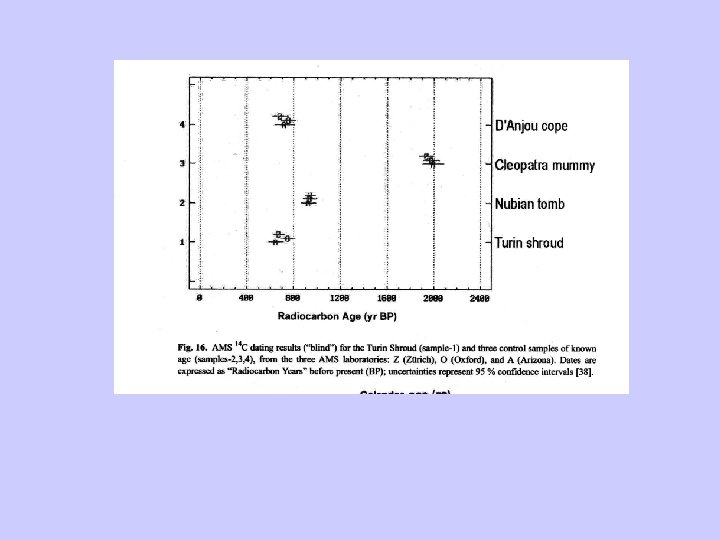
Radiocarbon dating-conclusion • Precise and fairly accurate (with adequate corrections). • Useful for the past ~50, 000 yr. • Widespread presence of C-bearing substrates. • Relatively small sample size (specially for AMS dates). • Contamination needs to be negligible.


Other Radio-isotopes • K-Ar – 40 K simultaneously decays to 40 Ca and 40 Ar(gas) – t 1/2=1. 3*109 yr (useful for rocks >500 kyr – Amount of 40 Ar is time-dependent – Problems: • Assumes that no 40 Ar enters or leaves the system • Limited to samples containing K • U-series

Other radio-isotopes • Uranium series – 236 U and 238 U decay to 226 Ra and 230 Th – U is included in carbonate lattice (e. g. , corals) – Age determined on the abundance of decay products – Problems: • Assumes a closed system • Assumes known initial conditions.

Thermo-luminescence (TL) • TL is light emitted from a crystal when it is heated. • TL signal depends on # e- trapped in the crystal. • Trapped e- originate from radioactive decay of surrounding minerals. • TL signal is proportional to time and intensity. • Useful between 100 yr and 106 yr

TL-Applications • Archaeological artifacts – Heating (>500 o. C) re-sets TL signal to zero – Used for dating pottery and baked sediments • Sediments – Exposure to sunlight re-sets the “clock” – Used for dating loess, sand dunes, river sand.

TL-Problems • Different response to ionization – # lattice defects – saturation • Incomplete re-setting • Water can absorb radiation • Unknown amount of ionization

Fission-Track Dating • 238 U • • can decay by spontaneous fission Small “tracks” are created on crystals (zircon, apatite, titanite) and volcanic glass. Track density is proportional to U-content and to time since the crystal formed. Useful for dating volcanic rocks (>200 kyr) Problem: tracks can “heal” over time