Data Recording and Significant Figures Accuracy and Precision








- Slides: 11

Data Recording and Significant Figures

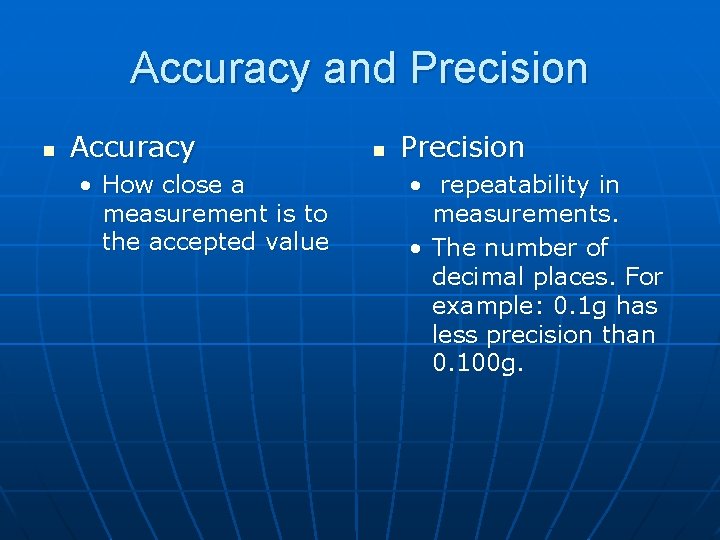
Accuracy and Precision n Accuracy • How close a measurement is to the accepted value n Precision • repeatability in measurements. • The number of decimal places. For example: 0. 1 g has less precision than 0. 100 g.

Recording measurements off of equipment. n estimate one more digit than the equipment provides.

Recording measurements continued n n Exception: with electronic equipment, the user is rounding off digits rather than adding them. For example, 2. 3456 g will be rounded to 2. 35 g. Round to nearest. 001 g.

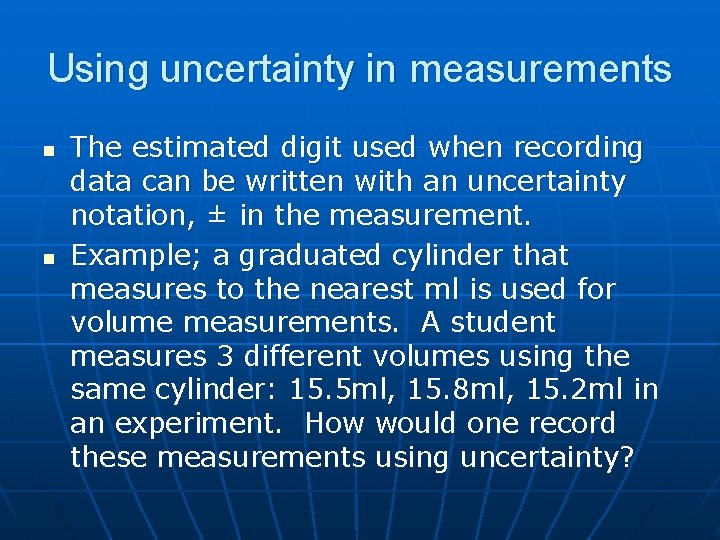
Using uncertainty in measurements n n The estimated digit used when recording data can be written with an uncertainty notation, ± in the measurement. Example; a graduated cylinder that measures to the nearest ml is used for volume measurements. A student measures 3 different volumes using the same cylinder: 15. 5 ml, 15. 8 ml, 15. 2 ml in an experiment. How would one record these measurements using uncertainty?

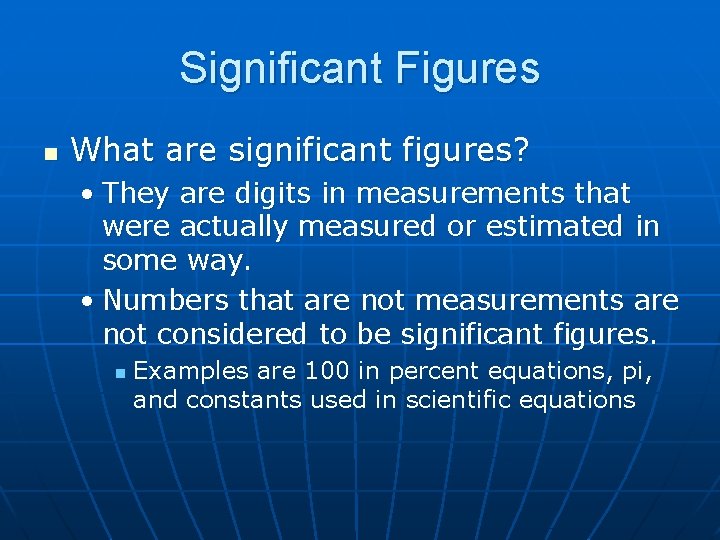
Significant Figures n What are significant figures? • They are digits in measurements that were actually measured or estimated in some way. • Numbers that are not measurements are not considered to be significant figures. n Examples are 100 in percent equations, pi, and constants used in scientific equations

Rules for using zeros. n Rule 1: Leading zeros are not significant. These are zeros which precede digits in decimal numbers. • Examples: 0. 045 g. , 0. 23 n Rule 2: Captive zeros are significant. These are any zeros that are in between non zero numbers. • Example 2, 013, 0. 0101, 100. 01

Use of zeros continued n Rule 3: Trailing zeros are not significant. These are zeros at the end of large numbers with no decimal point. • Examples: 100, 10, 2, 340 35, 000 n Scientific notation is used to remove the trailing zeros. Example: 35, 000 becomes 3. 50 x 104

Rules for rounding off measurements n Rule 1: when reducing the number of digits, look at the first digit that must be eliminated. • If it ends in a number greater than 5 round up. • If it ends in a number less than 5 round down. • If it ends exactly in 5, round to the nearest even number.

Rules for rounding in calculations n Addition and subtraction • Round off the final answer to the same number of decimal places as the measurement with the fewest decimal places. • Examples. 2. 34 g + 2. 35 g=7. 09 g this should be rounded to 7. 1 g

Rounding continued n Multiplication and division • The final answer has the same number of significant figures as the measurement with the fewest significant figures. • Example 150. ml x 2. 0 x 4. 14 = 1242 this must be rounded off to : n 1. 2 x 103