Data Quality Assurance Quality Control QAQC Requirements for










- Slides: 13

Data Quality Assurance/ Quality Control

QA/QC Requirements for RECAP Submittals • Data generated using rigorous analytical methods • Data must be analyte specific, and the identity and concentration confirmed • Method produced tangible raw data

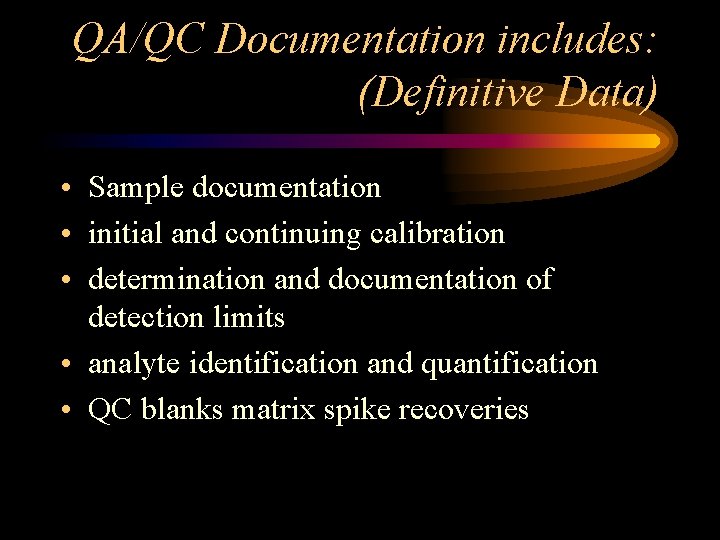
QA/QC Documentation includes: (Definitive Data) • Sample documentation • initial and continuing calibration • determination and documentation of detection limits • analyte identification and quantification • QC blanks matrix spike recoveries

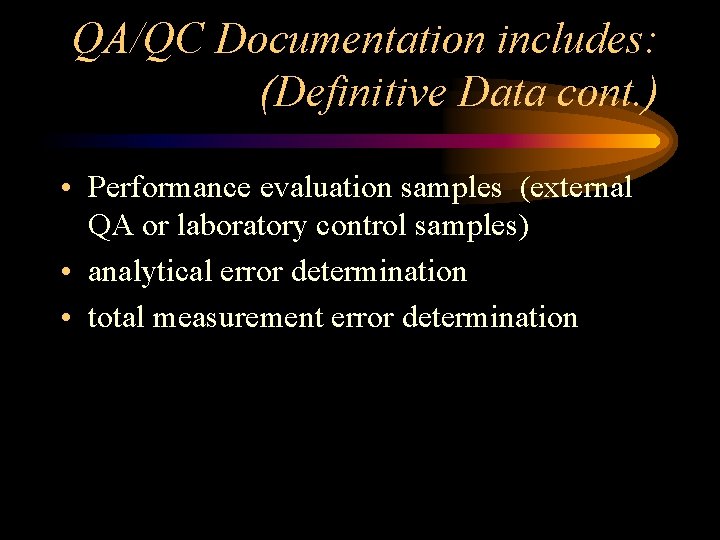
QA/QC Documentation includes: (Definitive Data cont. ) • Performance evaluation samples (external QA or laboratory control samples) • analytical error determination • total measurement error determination

Definitive Data Examples • • EPA 500 Series EPA 600 Series SW 846 methods Contract Laboratory Program (CLP) Statement of Work (SOW) methods

Example of an acceptable QA/QC set • • • 1 rinsate sample per 20 field samples 1 field blank per day 1 trip blank / ice chest of samples for VOA 1 field duplicate / 20 field samples 1 matrix spike/matrix spike duplicate from the site per 20 samples

Data Evaluation and Data Usability

Evaluate data with respect to: • • Analytical Method Sample Quantitation Limits Blank Samples Tentatively Identified Compounds (TICs)

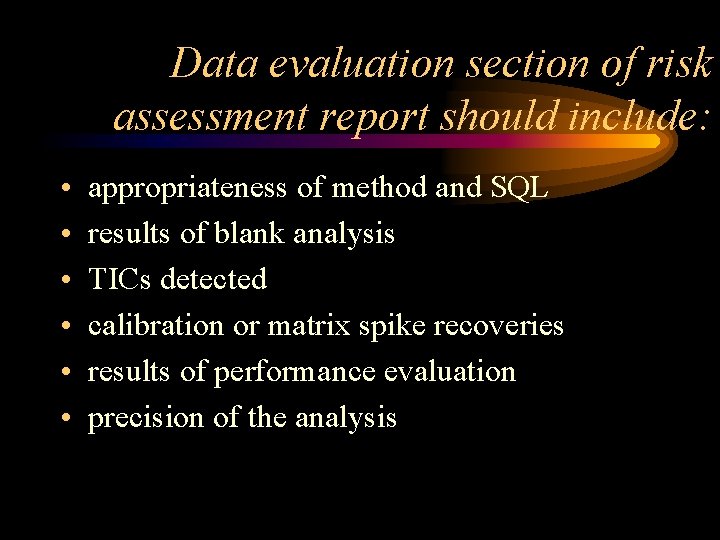
Data evaluation section of risk assessment report should include: • • • appropriateness of method and SQL results of blank analysis TICs detected calibration or matrix spike recoveries results of performance evaluation precision of the analysis

Historical data may be combined with current data if: • analytical methods and QA/QC, are similar for both data sets • constituents and concentrations detected in the historical data are consistent with the current data.

Historical data • Historical data of unknown quality may not be used in determining exposure or source concentrations • Analytical methods, sampling techniques, quantitation limits and QA/QC for the historical data shall be included in the risk assessment report

Historical data (cont. ) • If methods or concentrations are significantly different, the latest data set shall be used • the elimination of any data set shall be fully justified in the risk assessment report

Raw analytical data including chromatograms and additional QA/QC information may be requested by the Department on an as needed basis