Data Collection Notes How good are data Accuracy















- Slides: 16

Data Collection Notes How good are data?

Accuracy and Precision • Though the terms are frequently used interchangeably, accuracy and precision are two different things. – Accuracy: How close a measured value is to the true value. – Precision: How close a series of measurements are to each other.

• Accurate and precise: The knife thrower is precise (the knives are closely placed to one another) and accurate (the knives are where he wants it).

• Neither accurate nor precise: The knife thrower has neither placed the knives close to each other (making it imprecise) nor placed them where he wanted them (making them inaccurate).

• Precise, but not accurate: The knife thrower is precise (the knives are closely grouped) but not accurate (they are presumably not where he wanted them).

How can we tell if data are accurate? • In order to determine if data are accurate, we must first know what the actual value of the thing being measured is. • The accuracy of a measurement is expressed by the measurement’s “percent error”:

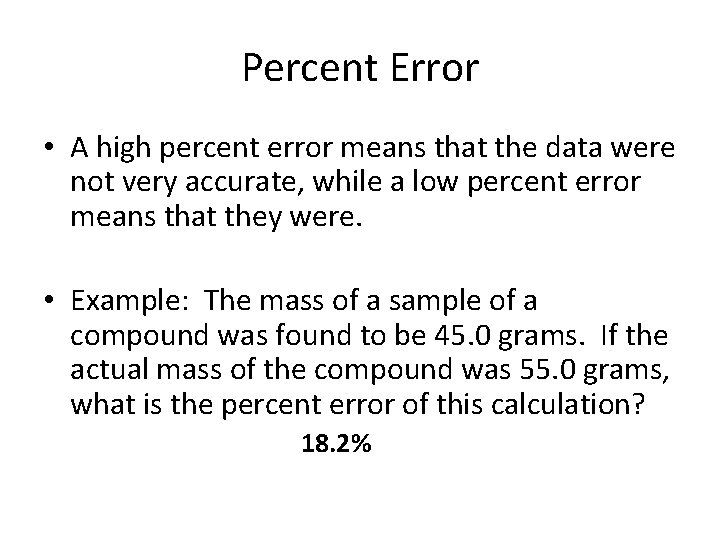
Percent Error • A high percent error means that the data were not very accurate, while a low percent error means that they were. • Example: The mass of a sample of a compound was found to be 45. 0 grams. If the actual mass of the compound was 55. 0 grams, what is the percent error of this calculation? 18. 2%

How can we tell if data are precise? • The number of decimal places that are shown in a measured value give us an idea of how precise they are. • For example, the measurement “ 18. 0 grams” is assumed to be precise to the nearest 0. 1 grams because if it wasn’t, we wouldn’t have gone to the trouble of writing the “. 0” at the end of the value. • The digits in a measured value that give us this kind of information are referred to as “significant digits” or “significant figures. ” Any digit that gives us useful information is said to be significant.

Sig Fig Rules: – Nonzero numbers in measurements are always significant. • The number “ 35 grams” has two significant figures and is precise to the nearest whole gram. – Zeros between nonzero numbers are always significant. • The number “ 202 grams” has three significant figures and is precise to the nearest whole gram. – Zeros to the left of all nonzero digits are never significant. They’re just placeholders. • The number “ 0. 02 grams” has one significant figure and is assumed to be precise to the nearest 0. 01 gram. • This rule is set up so that we get the same number of significant figures whether or not we use scientific notation.

Sig Fig Rules Continued: – Zeros to the right of all nonzero digits are only significant if there’s a decimal place explicitly shown. • The number “ 2. 00 grams” has three significant figures and is assumed to be precise to the nearest 0. 01 gram. • The number “ 0. 0020 grams” has two significant figures and is assumed to be precise to the nearest 0. 0001 gram. – When using scientific notation, only pay attention to the part of the value before the “x”. • The number “ 2. 0 x 102 grams” has two significant figures and is precise to the nearest 0. 1 x 102 grams.

PRACTICE! • How many sig figs in each? ü 122 ü 3 ü 12. 55 ü 4 ü 0. 0023 ü 2 ü 10 ü 1 ü 202. 02 ü 5 ü 99. 00 ü 4 ü 135. 980 ü 6 ü 120, 000 ü 2

Calculating with Sig Figs • When we do calculations using data, we need to make sure that our answers also reflect the value of the data that went into making them.

Calculating with Sig Figs • When adding and subtracting, the answer should be rounded to the last significant figure of the least precise value.

PRACTICE! • 325. 6 + 18 = • 344 • 1, 253. 67 – 17. 6 = • 1, 236. 1 • 221 – 16. 2 = • 205 • 1. 0236 + 0. 00547 = • 1. 0291

Calculating with Sig Figs • When multiplying and dividing, the answer should have the same number of significant figures as the value with the least number of significant figures. • 34. 0 grams / 10. 33 m. L = 3. 29 g/m. L – “ 34. 0 grams” has three significant figures and “ 10. 33 m. L” has four significant figures. Our answer, then, will be rounded to three significant figures.

PRACTICE! • 225. 36 grams x 23. 65 grams = • 5330. sq grams • 854 meters x 0. 0062 meters = • 5. 3 sq meters • 220 grams / 16. 2 cm 3 = • 14 g/cm 3 • 150. 0 / 10. 01= • 14. 99