DATA ANALYSIS Measurement Units Calculations Significant Figures Prefixes









- Slides: 17

DATA ANALYSIS Measurement; Units; Calculations; Significant Figures; Prefixes; Dimensional Analysis Neely's Chemistry

MEASUREMENT ØUsed to measure properties ØSI System based on the Metric System “Systeme International” ØBase Units & Derived Units Adopted by scientists in 1960 Neely's Chemistry

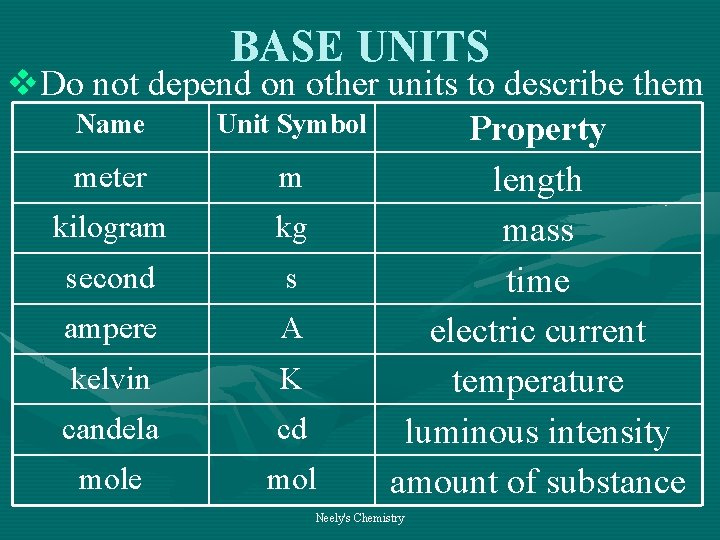
BASE UNITS v. Do not depend on other units to describe them Name Unit Symbol Property meter m length kilogram kg mass second s time ampere A electric current kelvin K temperature candela cd luminous intensity mole mol amount of substance Neely's Chemistry

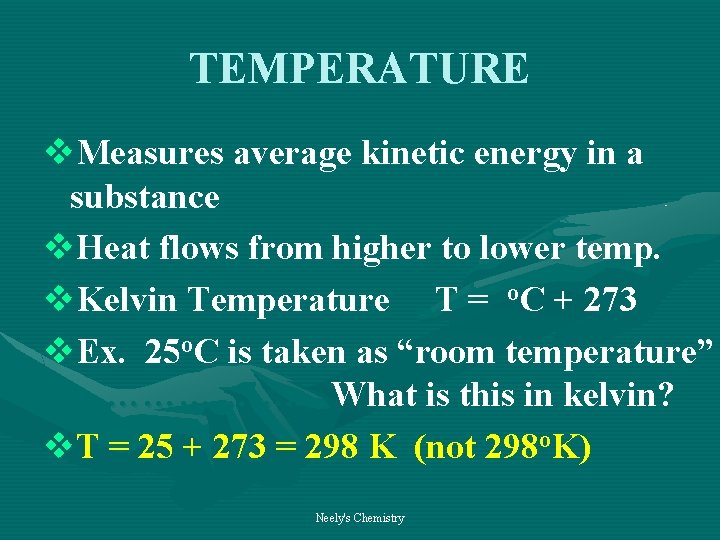
TEMPERATURE v. Measures average kinetic energy in a substance v. Heat flows from higher to lower temp. v. Kelvin Temperature T = o. C + 273 v. Ex. 25 o. C is taken as “room temperature” ………………. . . What is this in kelvin? v. T = 25 + 273 = 298 K (not 298 o. K) Neely's Chemistry

An experiment is performed at 378 K. What is this temperature in o. C? Ans. 105 o. C Neely's Chemistry

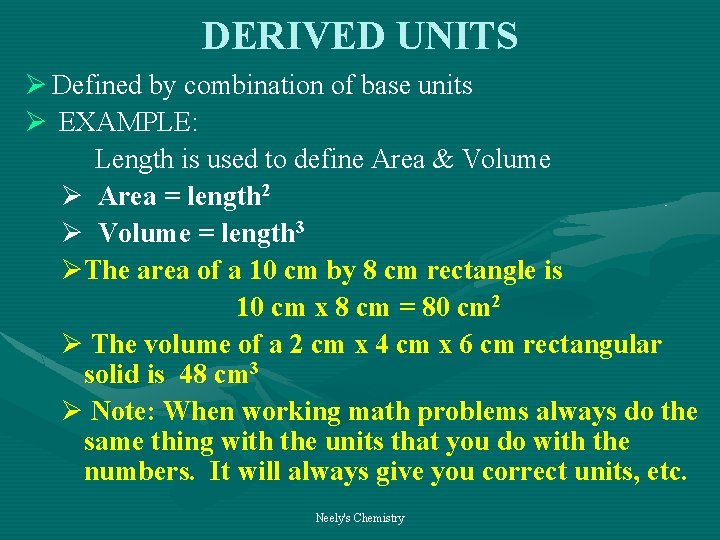
DERIVED UNITS Ø Defined by combination of base units Ø EXAMPLE: Length is used to define Area & Volume Ø Area = length 2 Ø Volume = length 3 ØThe area of a 10 cm by 8 cm rectangle is 10 cm x 8 cm = 80 cm 2 Ø The volume of a 2 cm x 4 cm x 6 cm rectangular solid is 48 cm 3 Ø Note: When working math problems always do the same thing with the units that you do with the numbers. It will always give you correct units, etc. Neely's Chemistry

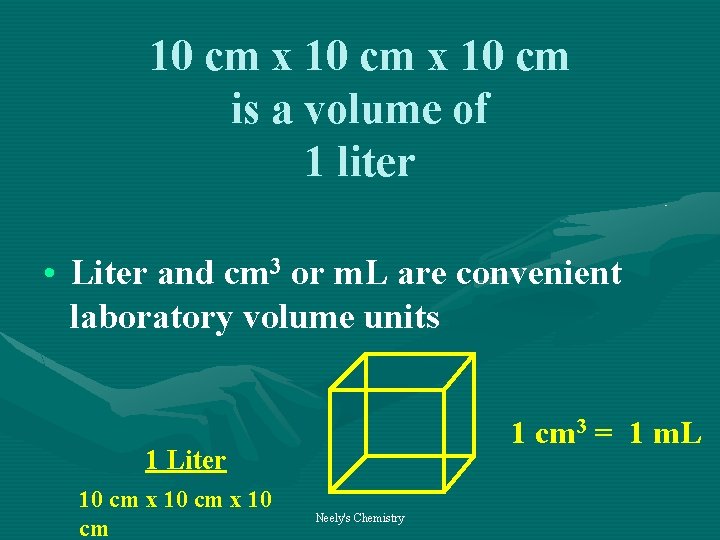
10 cm x 10 cm is a volume of 1 liter • Liter and cm 3 or m. L are convenient laboratory volume units 1 cm 3 = 1 m. L 1 Liter 10 cm x 10 cm Neely's Chemistry

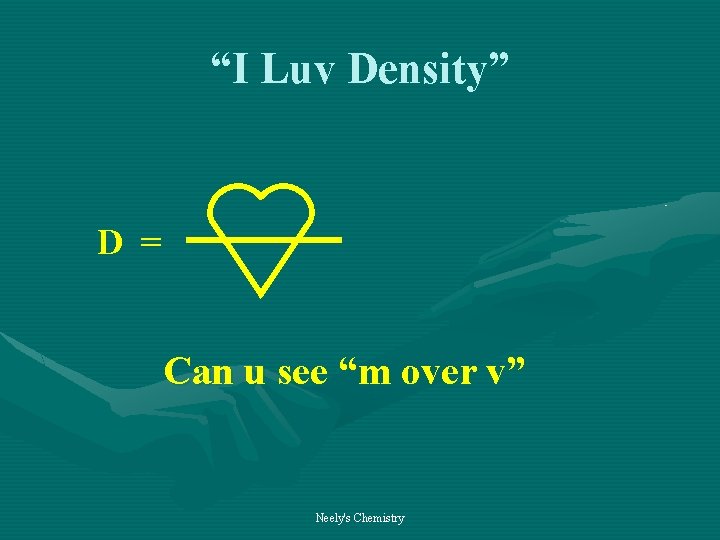
DENSITY an important derived unit • Defining Equation mass D = volume Neely's Chemistry

“I Luv Density” D = Can u see “m over v” Neely's Chemistry

A block of wood with a density of 0. 95 g/cm 3 is cut exactly in half. What is the resulting density of each half ? q. Density will be 0. 95 g/cc for each piece. q. Density is an intensive property. q. It does not depend on the size of the sample. Neely's Chemistry

Liquid Hg has a density of 13. 5 g/m. L. What is the mass of 20. 0 m. L of Hg? Neely's Chemistry

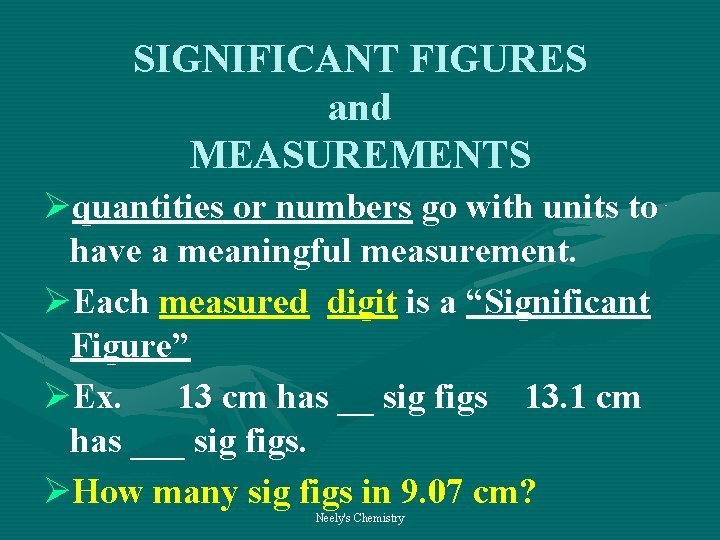
SIGNIFICANT FIGURES and MEASUREMENTS Øquantities or numbers go with units to have a meaningful measurement. ØEach measured digit is a “Significant Figure” ØEx. 13 cm has __ sig figs 13. 1 cm has ___ sig figs. ØHow many sig figs in 9. 07 cm? Neely's Chemistry

How Many Sig Figs in: Ø 10. 8 m ___ Ø 3 sig figs Ø 120. m ___ Ø 3 sig figs Ø 130 g ___ Ø 2 sig figs (the last zero implies but does not give a decimal) Neely's Chemistry

How many sig figs? • • • 0. 59 L 2 sig figs (0 merely there to “hold place”) 0. 0082 g 2 sig figs (all the zero’s “hold place”) 0. 0340 3 sig figs (only the 1 st 2 zero’s “hold place”. The last zero is a measured zero. ) Neely's Chemistry

Counting Significant Figures when a decimal is present ØCount from left to right ØStart counting sig fig digits when you get to the first non-zero ØAny zeros that occur after the first nonzero digit must count as significant Ø Ex. Sig figs in? a) 100. 0 ___ b) 0. 09140 ___ Neely's Chemistry

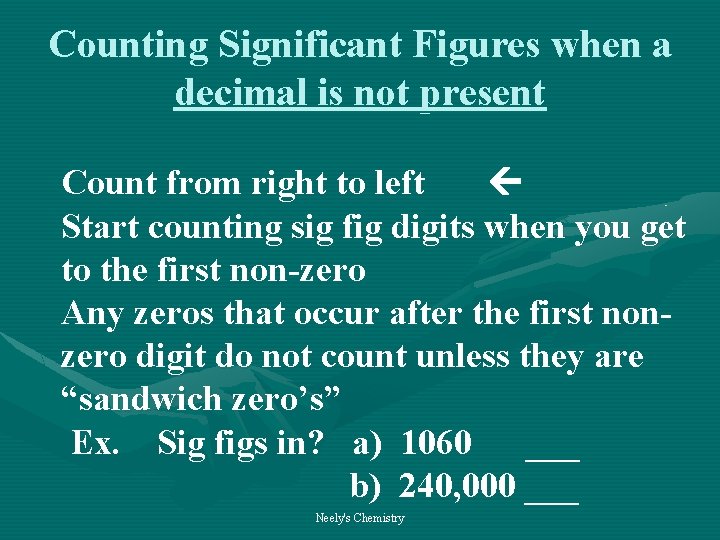
Counting Significant Figures when a decimal is not present Count from right to left Start counting sig fig digits when you get to the first non-zero Any zeros that occur after the first nonzero digit do not count unless they are “sandwich zero’s” Ex. Sig figs in? a) 1060 ___ b) 240, 000 ___ Neely's Chemistry

Complete the Significant Figure Work. Sheet Neely's Chemistry