Darunavirr Once Daily or Twice Daily in Treatment



- Slides: 7

Darunavir/r Once Daily or Twice Daily in Treatment Experienced ODIN Trial

Once-daily versus Twice-daily Darunavir in Treatment-Experienced ODIN: Study Design: ODIN • Background: Randomized, open-label phase 3 trial to compare once daily versus twice-daily dosing of ritonavir-boosted darunavir in treatmentexperienced patients with HIV infection • Inclusion Criteria (n = 590) - Age ≥ 18 - On stable antiretroviral regimen for >12 weeks - HIV RNA >1000 copies/m. L - CD 4 count >200 cells/mm 3 - No darunavir resistance-associated mutations • Treatment Arms - Darunavir 800 mg QD + RTV 100 mg QD + OBR* - Darunavir 600 mg BID + RTV 100 mg BID + OBR* Darunavir 800 mg QD + Ritonavir 100 mg QD + OBR (n = 294) Darunavir 600 mg BID + Ritonavir 100 mg BID + OBR (n = 296) ODIN = Once-daily Darunavir In treatment-experie. Nced patients *OBR = Optimized background regimen: ≥ 2 nucleoside reverse transcriptase inhibitors, investigator-selected Source: Cahn P, et al. AIDS. 2011; 25: 929 -39.

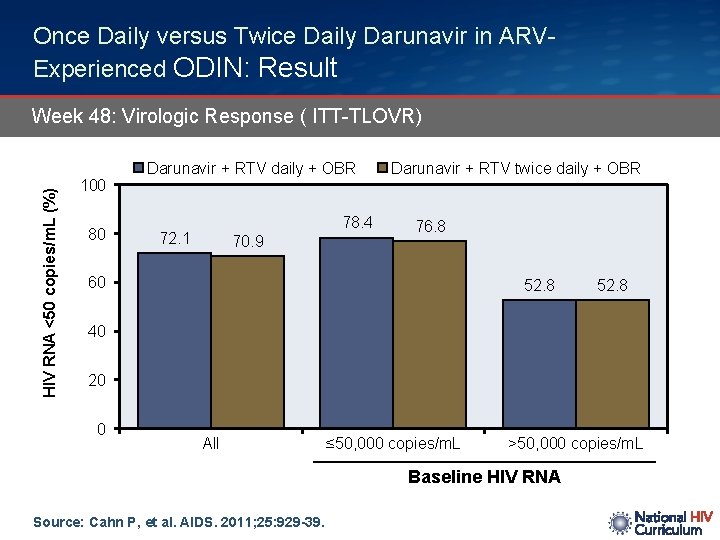
Once Daily versus Twice Daily Darunavir in ARVExperienced ODIN: Result HIV RNA <50 copies/m. L (%) Week 48: Virologic Response ( ITT-TLOVR) 100 80 Darunavir + RTV daily + OBR 78. 4 72. 1 70. 9 Darunavir + RTV twice daily + OBR 76. 8 60 52. 8 40 20 0 All ≤ 50, 000 copies/m. L >50, 000 copies/m. L Baseline HIV RNA Source: Cahn P, et al. AIDS. 2011; 25: 929 -39.

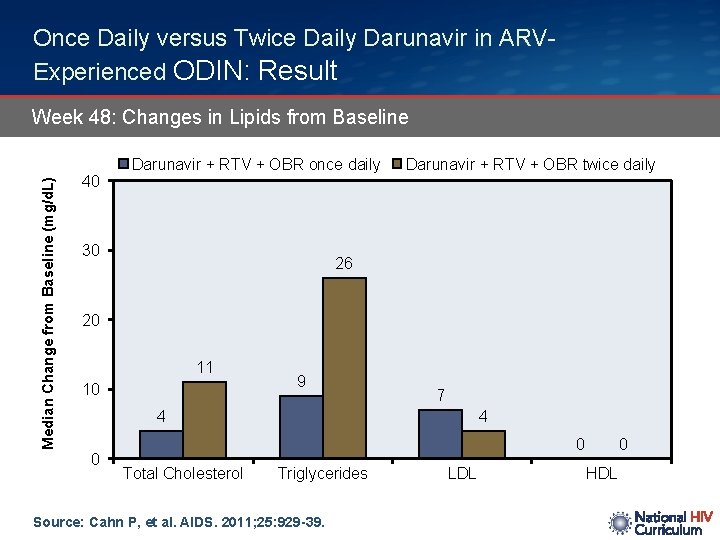
Once Daily versus Twice Daily Darunavir in ARVExperienced ODIN: Result Median Change from Baseline (mg/d. L) Week 48: Changes in Lipids from Baseline 40 Darunavir + RTV + OBR once daily 30 Darunavir + RTV + OBR twice daily 26 20 11 10 9 7 4 0 Total Cholesterol Triglycerides Source: Cahn P, et al. AIDS. 2011; 25: 929 -39. LDL 0 HDL

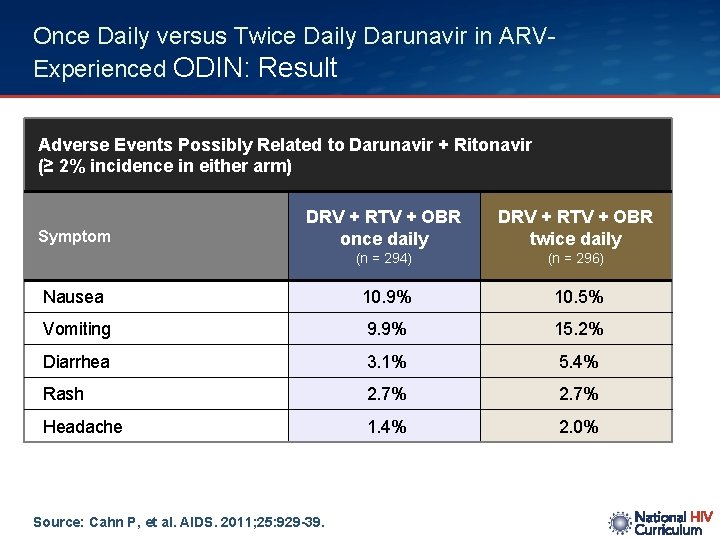
Once Daily versus Twice Daily Darunavir in ARVExperienced ODIN: Result Adverse Events Possibly Related to Darunavir + Ritonavir (≥ 2% incidence in either arm) DRV + RTV + OBR once daily DRV + RTV + OBR twice daily (n = 294) (n = 296) Nausea 10. 9% 10. 5% Vomiting 9. 9% 15. 2% Diarrhea 3. 1% 5. 4% Rash 2. 7% Headache 1. 4% 2. 0% Symptom Source: Cahn P, et al. AIDS. 2011; 25: 929 -39.

Once Daily versus Twice Daily Darunavir in ARVExperienced ODIN: Conclusions Conclusion: “Once-daily DRV/r 800/100 mg was non-inferior in virologic response to twice-daily DRV/r 600/100 mg at 48 weeks in treatmentexperienced patients with no DRV RAMs, and with a more favorable lipid profile. These findings support use of once-daily DRV/r in this population. ” Source: Cahn P, et al. AIDS. 2011; 25: 929 -39.

Acknowledgment The National HIV Curriculum is an AIDS Education and Training Center (AETC) Program resource funded by the United States Health Resources and Services Administration. The project is led by the University of Washington and the AETC National Coordinating Resource Center. The content in this slide set does not represent the official views of the U. S. Department of Health and Human Services, Health Resources & Services Administration.