Dallas Texas Key Concepts of Type 2 Diabetes

Dallas, Texas Key Concepts of Type 2 Diabetes Mellitus Vivian A. Fonseca, MD, FRCP Professor of Medicine and Pharmacology Tullis Tulane Alumni Chair in Diabetes Chief, Section of Endocrinology Tulane University School of Medicine New Orleans, Louisiana Jonathan D. Leffert, MD, FACP, FACE, ECNU Managing Partner, North Texas Endocrine Center President, American Association of Clinical Endocrinologists Dallas, Texas
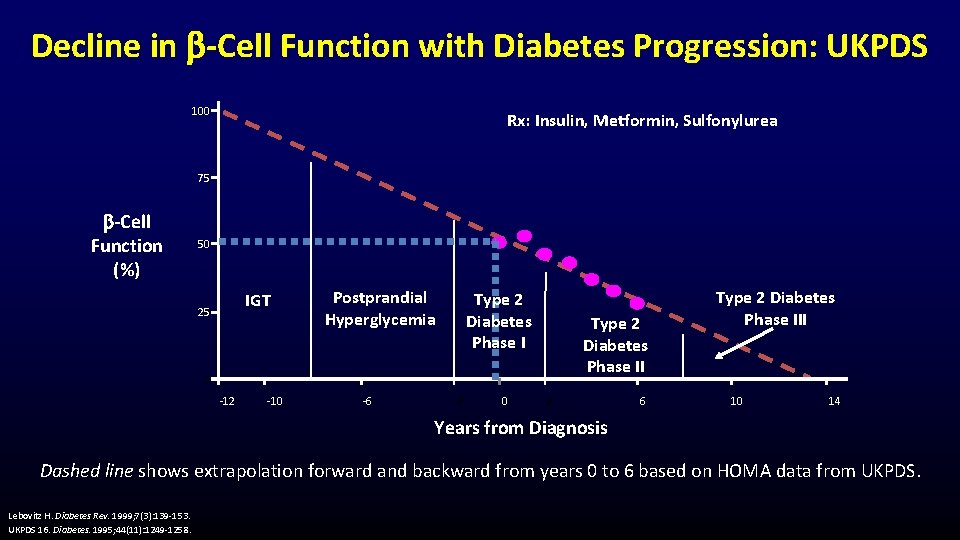
Decline in -Cell Function with Diabetes Progression: UKPDS 100 Rx: Insulin, Metformin, Sulfonylurea 75 -Cell Function (%) 50 IGT 25 Postprandial Hyperglycemia Type 2 Diabetes Phase II 0 -12 -10 -6 -2 0 2 6 Type 2 Diabetes Phase III 10 14 Years from Diagnosis Dashed line shows extrapolation forward and backward from years 0 to 6 based on HOMA data from UKPDS. Lebovitz H. Diabetes Rev. 1999; 7(3): 139 -153. UKPDS 16. Diabetes. 1995; 44(11): 1249 -1258.
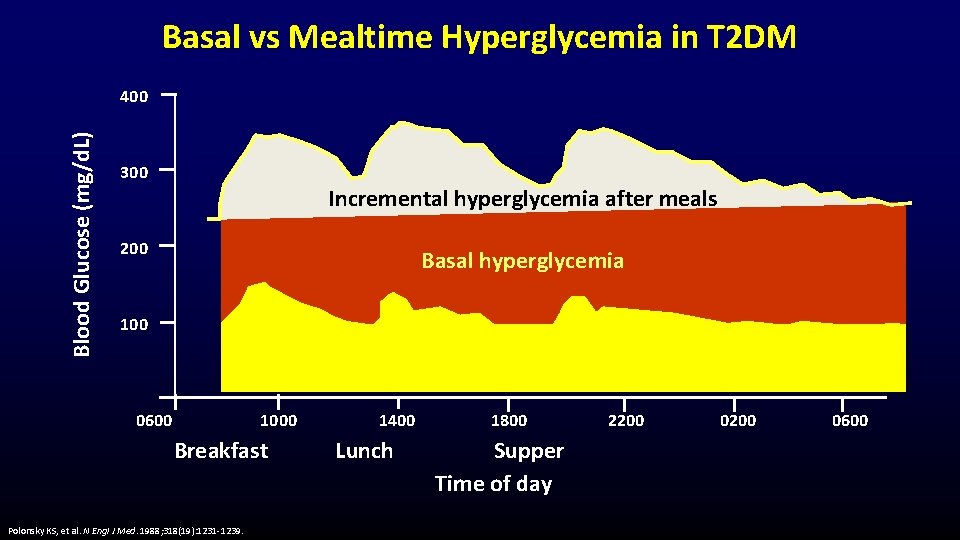
Basal vs Mealtime Hyperglycemia in T 2 DM Blood Glucose (mg/d. L) 400 300 Incremental hyperglycemia after meals 200 Basal hyperglycemia 100 0600 1000 Breakfast Polonsky KS, et al. N Engl J Med. 1988; 318(19): 1231 -1239. 1400 Lunch 1800 Supper Time of day 2200 0600

ADA and AACE Glycemic Targets Glycemic Control Targets Test ADA AACE Hb. A 1 c <7% ≤ 6. 5%3 FPG 80 -130 mg/d. L <110 mg/d. L 3 PPG <180 mg/d. L (measured within 1 to 2 hours after the start of a meal) <140 mg/d. L 3 (2 -hour value) Hb. A 1 C target should be individualized based on numerous factors, including age, life expectancy, comorbid conditions, duration of diabetes, risk of hypoglycemia or adverse consequences from hypoglycemia, patient motivation, and adherence 1, 2 AACE, American Association of Clinical Endocrinologists; ADA, American Diabetes Association; FPG, fasting plasma glucose; PPG, postprandial glucose. 1. American Diabetes Association. Diabetes Care. 2017; 40(suppl 1): S 1 -S 135. 2. Garber AJ, et al. Endocr Pract. 2017; 23(2): 207 -238. 3. Handelsman Y et al. Endocr Pract. 2015; 21(suppl 1): 1 -87.

When To Start Insulin in T 2 DM • Patients with – hyperglycemic emergencies – symptomatic hyperglycemia and/or markedly high Hb. A 1 c – hepatic or renal disease – coronary artery disease, ↑ triglyceride level • • When combination oral/injectable agents become inadequate Unacceptable side effects of oral/injectable agents Patient wants more flexibility Special circumstances (ie, steroid use, infection, pregnancy) Holman RR, et al. NEJM. 2009; 361(18): 1736 -1747. Lebovitz HE. Diabetes Rev. 1999; 7(3): 139 -153.
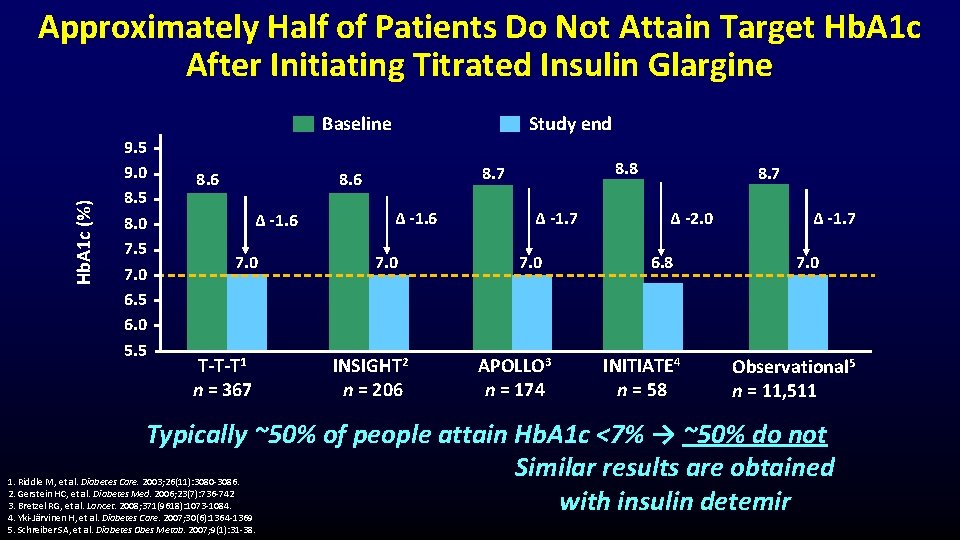
Hb. A 1 c (%) Approximately Half of Patients Do Not Attain Target Hb. A 1 c After Initiating Titrated Insulin Glargine 9. 5 9. 0 8. 5 8. 0 7. 5 7. 0 6. 5 6. 0 5. 5 Baseline 8. 6 Study end ∆ -1. 6 7. 0 T-T-T 1 n = 367 8. 8 8. 7 8. 6 ∆ -1. 6 7. 0 INSIGHT 2 n = 206 ∆ -1. 7 7. 0 APOLLO 3 n = 174 8. 7 ∆ -2. 0 6. 8 INITIATE 4 n = 58 ∆ -1. 7 7. 0 Observational 5 n = 11, 511 Typically ~50% of people attain Hb. A 1 c <7% → ~50% do not Similar results are obtained with insulin detemir 1. Riddle M, et al. Diabetes Care. 2003; 26(11): 3080 -3086. 2. Gerstein HC, et al. Diabetes Med. 2006; 23(7): 736 -742 3. Bretzel RG, et al. Lancet. 2008; 371(9618): 1073 -1084. 4. Yki-Järvinen H, et al. Diabetes Care. 2007; 30(6): 1364 -1369 5. Schreiber SA, et al. Diabetes Obes Metab. 2007; 9(1): 31 -38.
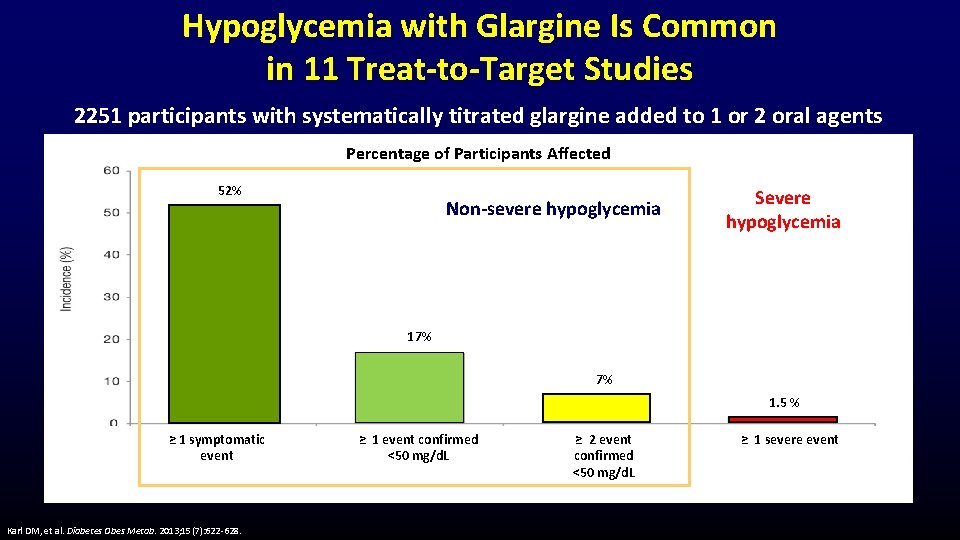
Hypoglycemia with Glargine Is Common in 11 Treat-to-Target Studies 2251 participants with systematically titrated glargine added to 1 or 2 oral agents Percentage of Participants Affected 52% Non-severe hypoglycemia Severe hypoglycemia 17% 7% 1. 5 % ≥ 1 symptomatic event Karl DM, et al. Diabetes Obes Metab. 2013; 15(7): 622 -628. ≥ 1 event confirmed <50 mg/d. L ≥ 2 event confirmed <50 mg/d. L ≥ 1 severe event
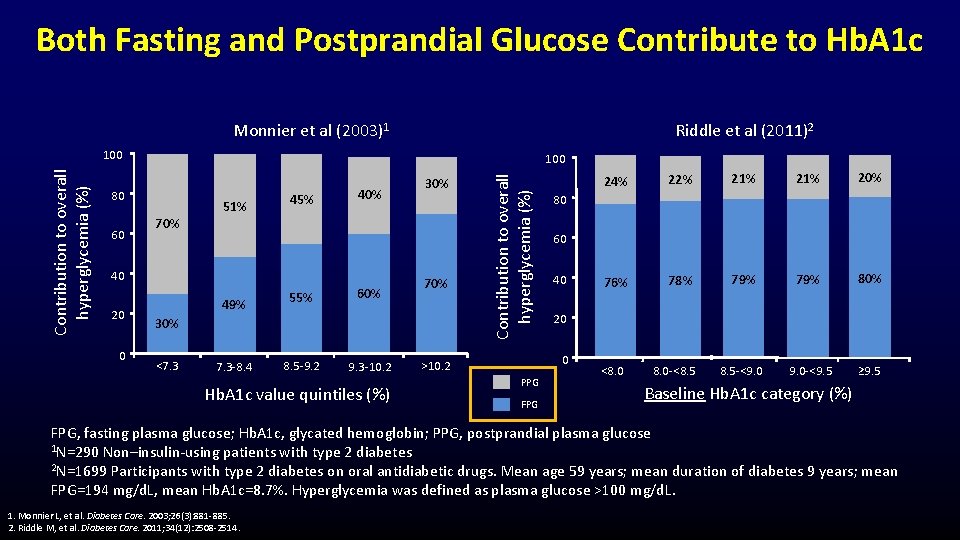
Both Fasting and Postprandial Glucose Contribute to Hb. A 1 c Monnier et al (2003)1 Riddle et al (2011)2 100 80 60 70% 51% 45% 40 20 0 49% 55% 7. 3 -8. 4 8. 5 -9. 2 60% 30% 70% 30% <7. 3 9. 3 -10. 2 Hb. A 1 c value quintiles (%) Contribution to overall hyperglycemia (%) 100 PPG FPG 22% 21% 20% 76% 78% 79% 80% 8. 5 -<9. 0 -<9. 5 ≥ 9. 5 80 60 40 20 0 >10. 2 24% <8. 0 -<8. 5 Baseline Hb. A 1 c category (%) FPG, fasting plasma glucose; Hb. A 1 c, glycated hemoglobin; PPG, postprandial plasma glucose 1 N=290 Non–insulin-using patients with type 2 diabetes 2 N=1699 Participants with type 2 diabetes on oral antidiabetic drugs. Mean age 59 years; mean duration of diabetes 9 years; mean FPG=194 mg/d. L, mean Hb. A 1 c=8. 7%. Hyperglycemia was defined as plasma glucose >100 mg/d. L. 1. Monnier L, et al. Diabetes Care. 2003; 26(3): 881 -885. 2. Riddle M, et al. Diabetes Care. 2011; 34(12): 2508 -2514.
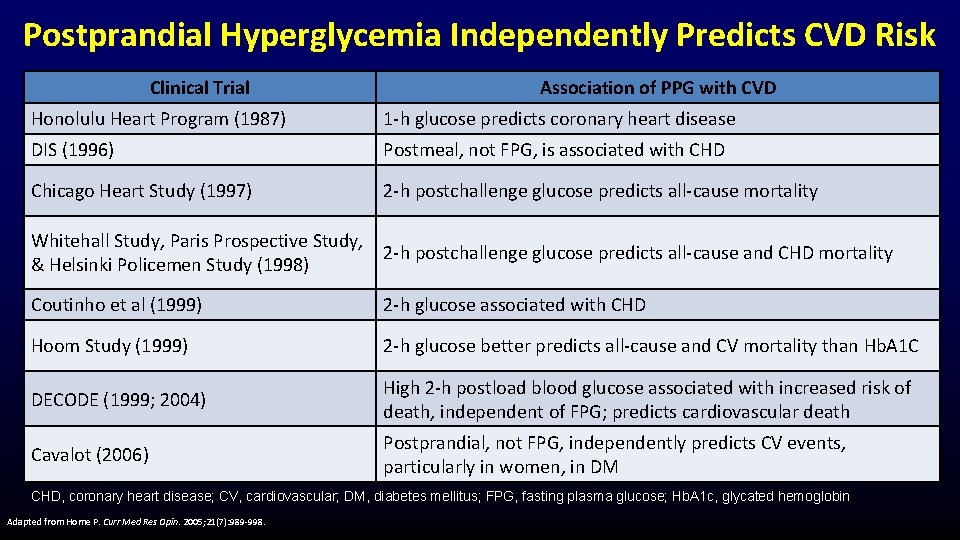
Postprandial Hyperglycemia Independently Predicts CVD Risk Clinical Trial Association of PPG with CVD Honolulu Heart Program (1987) 1 -h glucose predicts coronary heart disease DIS (1996) Postmeal, not FPG, is associated with CHD Chicago Heart Study (1997) 2 -h postchallenge glucose predicts all-cause mortality Whitehall Study, Paris Prospective Study, 2 -h postchallenge glucose predicts all-cause and CHD mortality & Helsinki Policemen Study (1998) Coutinho et al (1999) 2 -h glucose associated with CHD Hoom Study (1999) 2 -h glucose better predicts all-cause and CV mortality than Hb. A 1 C DECODE (1999; 2004) High 2 -h postload blood glucose associated with increased risk of death, independent of FPG; predicts cardiovascular death Cavalot (2006) Postprandial, not FPG, independently predicts CV events, particularly in women, in DM CHD, coronary heart disease; CV, cardiovascular; DM, diabetes mellitus; FPG, fasting plasma glucose; Hb. A 1 c, glycated hemoglobin Adapted from Home P. Curr Med Res Opin. 2005; 21(7): 989 -998.
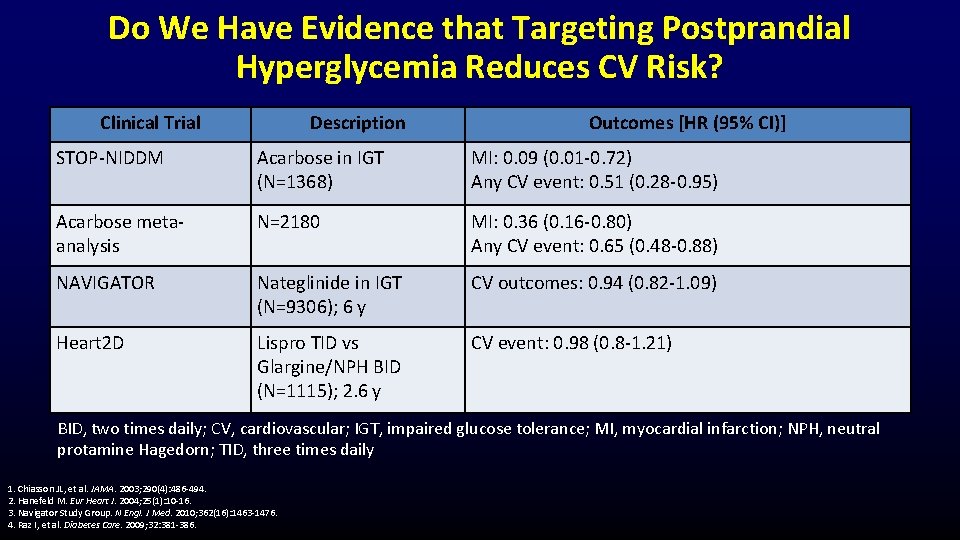
Do We Have Evidence that Targeting Postprandial Hyperglycemia Reduces CV Risk? Clinical Trial Description Outcomes [HR (95% CI)] STOP-NIDDM Acarbose in IGT (N=1368) MI: 0. 09 (0. 01 -0. 72) Any CV event: 0. 51 (0. 28 -0. 95) Acarbose metaanalysis N=2180 MI: 0. 36 (0. 16 -0. 80) Any CV event: 0. 65 (0. 48 -0. 88) NAVIGATOR Nateglinide in IGT (N=9306); 6 y CV outcomes: 0. 94 (0. 82 -1. 09) Heart 2 D Lispro TID vs Glargine/NPH BID (N=1115); 2. 6 y CV event: 0. 98 (0. 8 -1. 21) BID, two times daily; CV, cardiovascular; IGT, impaired glucose tolerance; MI, myocardial infarction; NPH, neutral protamine Hagedorn; TID, three times daily 1. Chiasson JL, et al. JAMA. 2003; 290(4): 486 -494. 2. Hanefeld M. Eur Heart J. 2004; 25(1): 10 -16. 3. Navigator Study Group. N Engl. J Med. 2010; 362(16): 1463 -1476. 4. Raz I, et al. Diabetes Care. 2009; 32: 381 -386.

Innovations in Basal Insulin Analogs Vivian A. Fonseca, MD, FRCP Professor of Medicine and Pharmacology Tullis Tulane Alumni Chair in Diabetes Chief, Section of Endocrinology Tulane University School of Medicine New Orleans, Louisiana Jonathan D. Leffert, MD, FACP, FACE, ECNU Managing Partner, North Texas Endocrine Center President, American Association of Clinical Endocrinologists Dallas, Texas
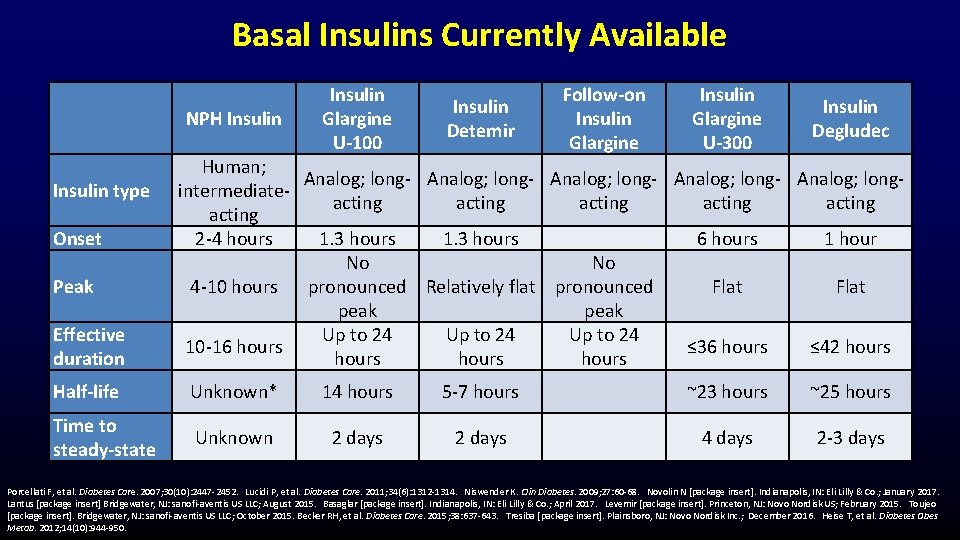
Basal Insulins Currently Available NPH Insulin type Onset Peak Effective duration Insulin Glargine U-100 Insulin Detemir Follow-on Insulin Glargine U-300 Insulin Degludec Human; Analog; long- Analog; longintermediateacting acting 2 -4 hours 1. 3 hours 6 hours 1 hour No No 4 -10 hours pronounced Relatively flat pronounced Flat peak Up to 24 10 -16 hours ≤ 36 hours ≤ 42 hours Half-life Unknown* 14 hours 5 -7 hours ~23 hours ~25 hours Time to steady-state Unknown 2 days 4 days 2 -3 days Porcellati F, et al. Diabetes Care. 2007; 30(10): 2447 -2452. Lucidi P, et al. Diabetes Care. 2011; 34(6): 1312 -1314. Niswender K. Clin Diabetes. 2009; 27: 60 -68. Novolin N [package insert]. Indianapolis, IN: Eli Lilly & Co. ; January 2017. Lantus [package insert] Bridgewater, NJ: sanofi-aventis US LLC; August 2015. Basaglar [package insert]. Indianapolis, IN: Eli Lilly & Co. ; April 2017. Levemir [package insert]. Princeton, NJ: Novo Nordisk US; February 2015. Toujeo [package insert]. Bridgewater, NJ: sanofi-aventis US LLC; October 2015. Becker RH, et al. Diabetes Care. 2015; 38: 637 -643. Tresiba [package insert]. Plainsboro, NJ: Novo Nordisk Inc. ; December 2016. Heise T, et al. Diabetes Obes Metab. 2012; 14(10): 944 -950.
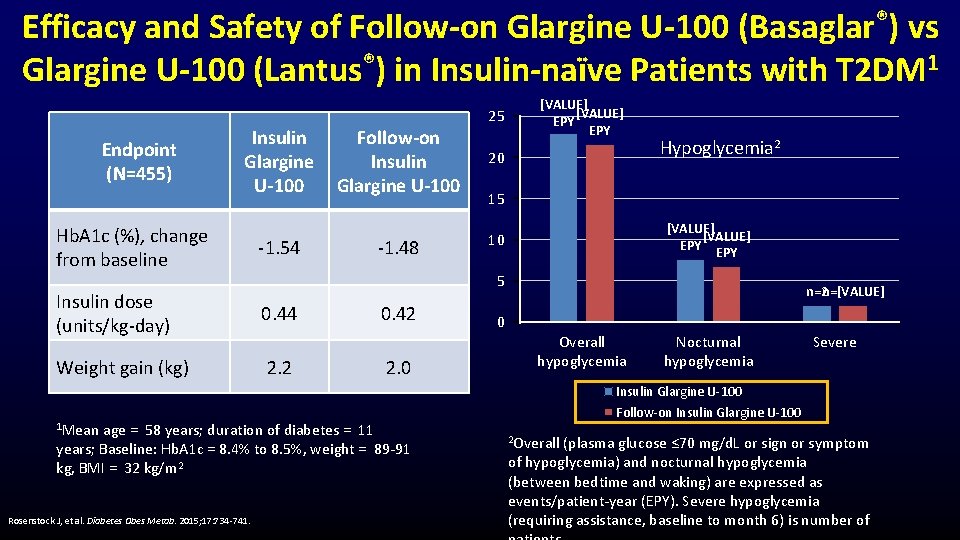
Efficacy and Safety of Follow-on Glargine U-100 (Basaglar®) vs Glargine U-100 (Lantus®) in Insulin-naïve Patients with T 2 DM 1 Endpoint (N=455) Insulin Glargine U-100 Hb. A 1 c (%), change from baseline -1. 54 -1. 48 0. 44 Weight gain (kg) 2. 2 0. 42 2. 0 age = 58 years; duration of diabetes = 11 years; Baseline: Hb. A 1 c = 8. 4% to 8. 5%, weight = 89 -91 kg, BMI = 32 kg/m 2 Rosenstock J, et al. Diabetes Obes Metab. 2015; 17: 734 -741. [VALUE] EPY 20 Hypoglycemia 2 15 [VALUE] EPY 10 5 Insulin dose (units/kg-day) 1 Mean Follow-on Insulin Glargine U-100 25 n=2 n=[VALUE] 0 Overall hypoglycemia Nocturnal hypoglycemia Severe Insulin Glargine U-100 Follow-on Insulin Glargine U-100 2 Overall (plasma glucose ≤ 70 mg/d. L or sign or symptom of hypoglycemia) and nocturnal hypoglycemia (between bedtime and waking) are expressed as events/patient-year (EPY). Severe hypoglycemia (requiring assistance, baseline to month 6) is number of
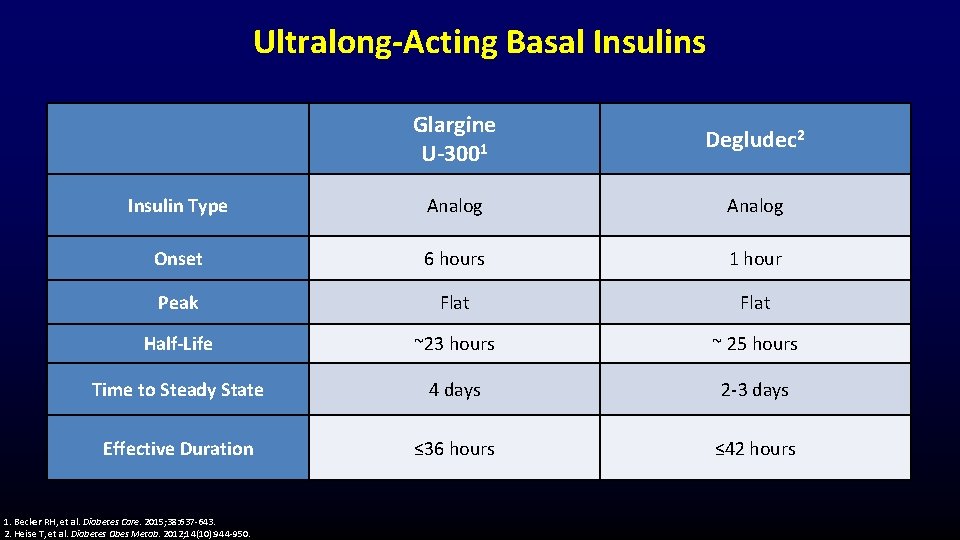
Ultralong-Acting Basal Insulins Glargine U-3001 Degludec 2 Insulin Type Analog Onset 6 hours 1 hour Peak Flat Half-Life ~23 hours ~ 25 hours Time to Steady State 4 days 2 -3 days Effective Duration ≤ 36 hours ≤ 42 hours 1. Becker RH, et al. Diabetes Care. 2015; 38: 637 -643. 2. Heise T, et al. Diabetes Obes Metab. 2012; 14(10): 944 -950.
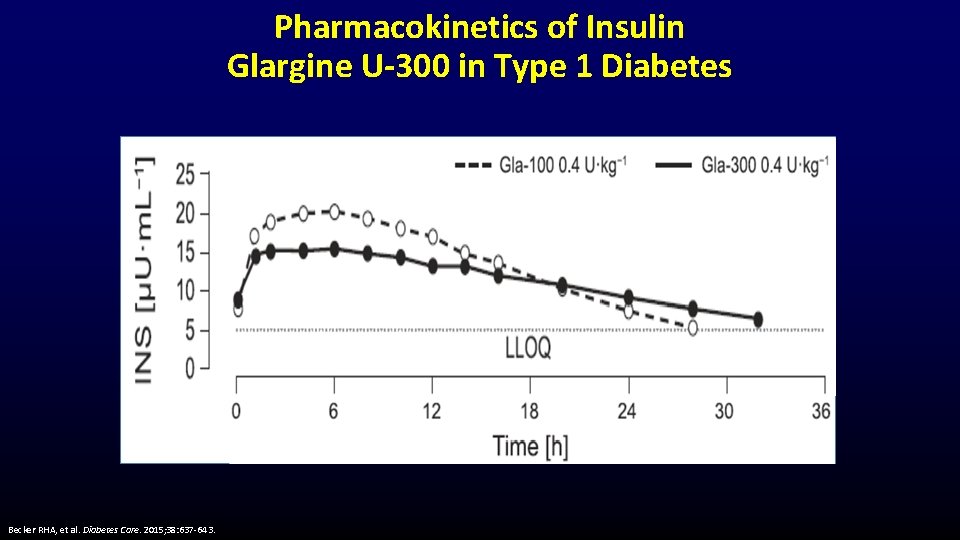
Pharmacokinetics of Insulin Glargine U-300 in Type 1 Diabetes Becker RHA, et al. Diabetes Care. 2015; 38: 637 -643.

Insulin Glargine U-300: EDITION Program Anderson JE. J Fam Pract. 2016; 65(10 Suppl): S 23 -S 28.
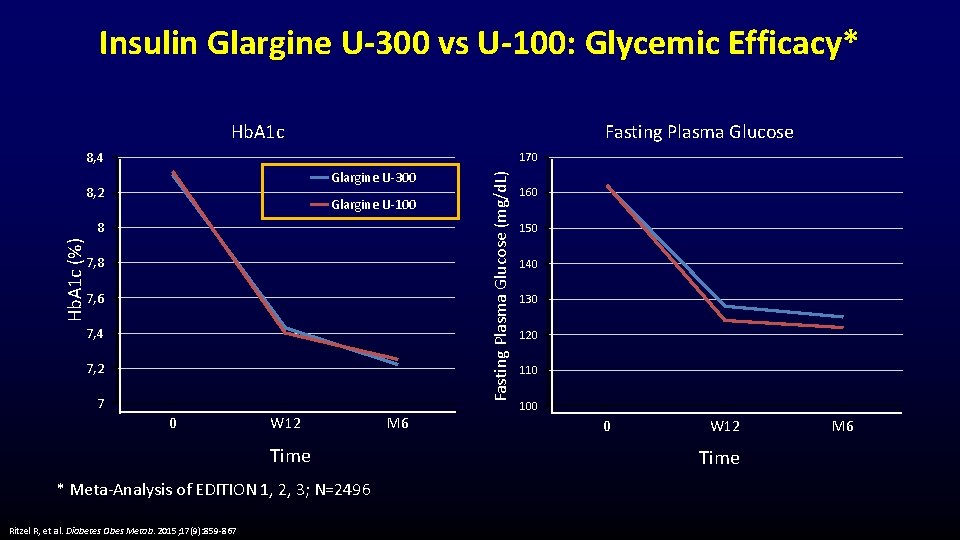
Insulin Glargine U-300 vs U-100: Glycemic Efficacy* Hb. A 1 c Fasting Plasma Glucose 8, 4 Glargine U-300 8, 2 Glargine U-100 Hb. A 1 c (%) 8 7, 6 7, 4 7, 2 7 0 W 12 Time * Meta-Analysis of EDITION 1, 2, 3; N=2496 Ritzel R, et al. Diabetes Obes Metab. 2015; 17(9): 859 -867 M 6 Fasting Plasma Glucose (mg/d. L) 170 160 150 140 130 120 110 100 0 W 12 Time M 6
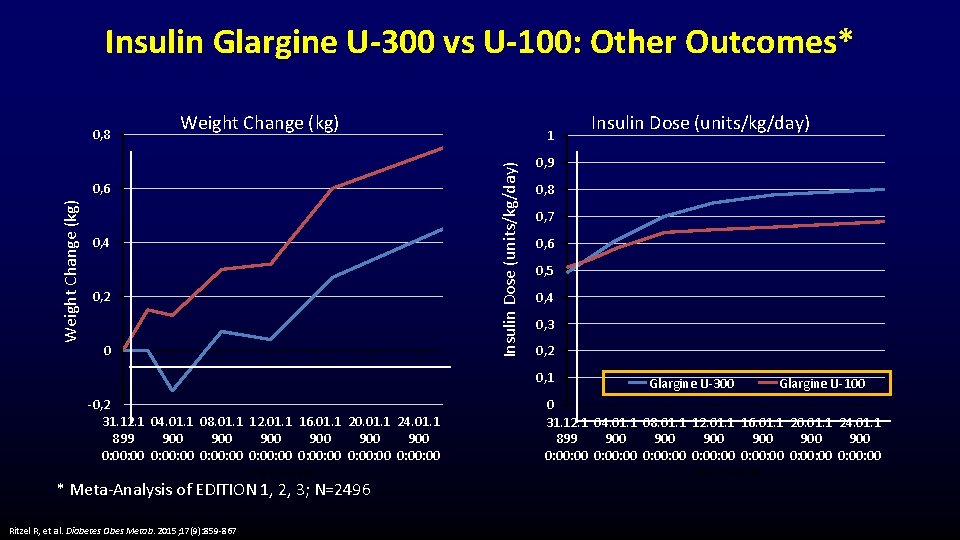
Insulin Glargine U-300 vs U-100: Other Outcomes* Weight Change (kg) 0, 6 Weight Change (kg) 1 Insulin Dose (units/kg/day) 0, 8 0, 4 0, 2 0 0, 9 0, 8 0, 7 0, 6 0, 5 0, 4 0, 3 0, 2 0, 1 -0, 2 31. 12. 1 04. 01. 1 08. 01. 1 12. 01. 1 16. 01. 1 20. 01. 1 24. 01. 1 899 900 900 900 0: 00: 00 0: 00: 00 Time (weeks) * Meta-Analysis of EDITION 1, 2, 3; N=2496 Ritzel R, et al. Diabetes Obes Metab. 2015; 17(9): 859 -867 Insulin Dose (units/kg/day) Glargine U-300 Glargine U-100 0 31. 12. 1 04. 01. 1 08. 01. 1 12. 01. 1 16. 01. 1 20. 01. 1 24. 01. 1 899 900 900 900 0: 00: 00 0: 00: 00 Time (weeks)
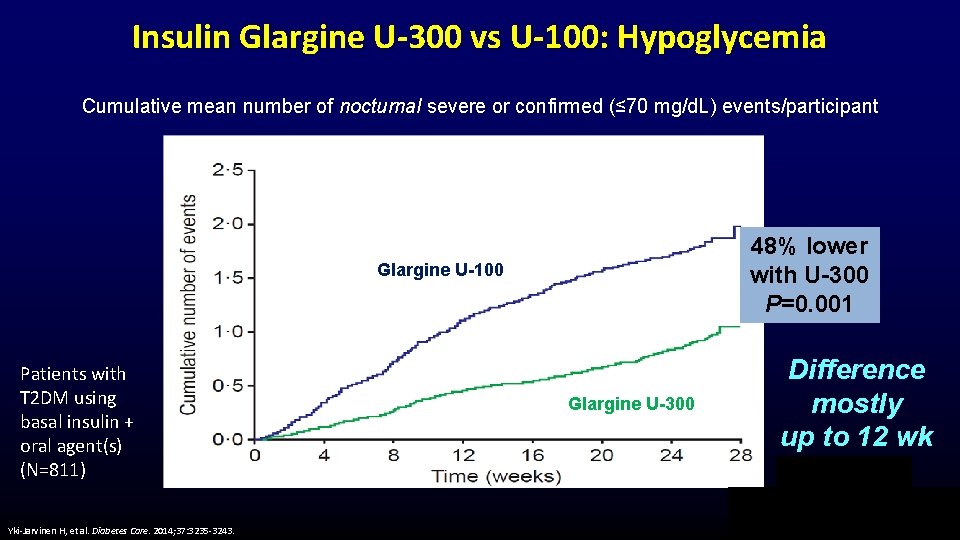
Insulin Glargine U-300 vs U-100: Hypoglycemia Cumulative mean number of nocturnal severe or confirmed (≤ 70 mg/d. L) events/participant 48% lower with U-300 P=0. 001 Glargine U-100 Patients with T 2 DM using basal insulin + oral agent(s) (N=811) Yki-Jarvinen H, et al. Diabetes Care. 2014; 37: 3235 -3243. Glargine U-300 Difference mostly up to 12 wk
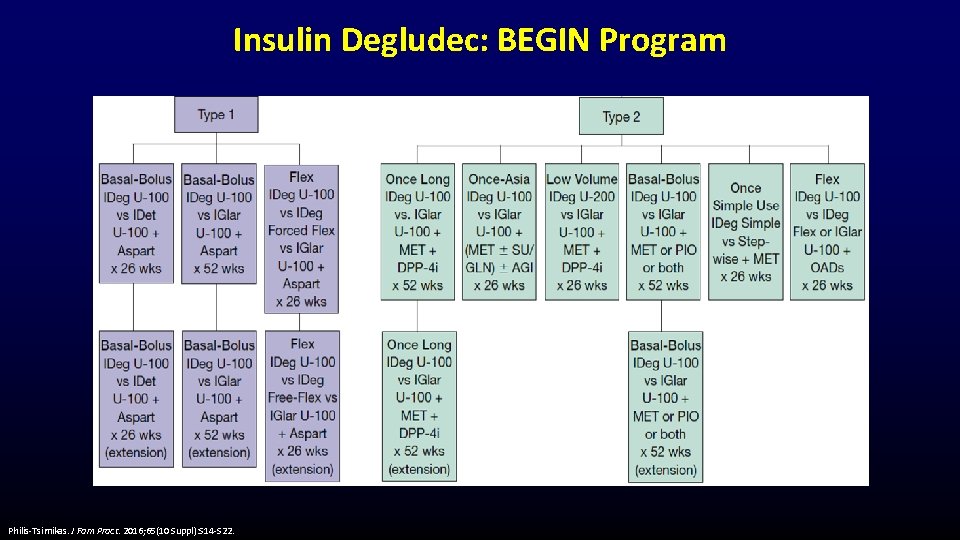
Insulin Degludec: BEGIN Program Philis-Tsimikas. J Fam Pract. 2016; 65(10 Suppl): S 14 -S 22.
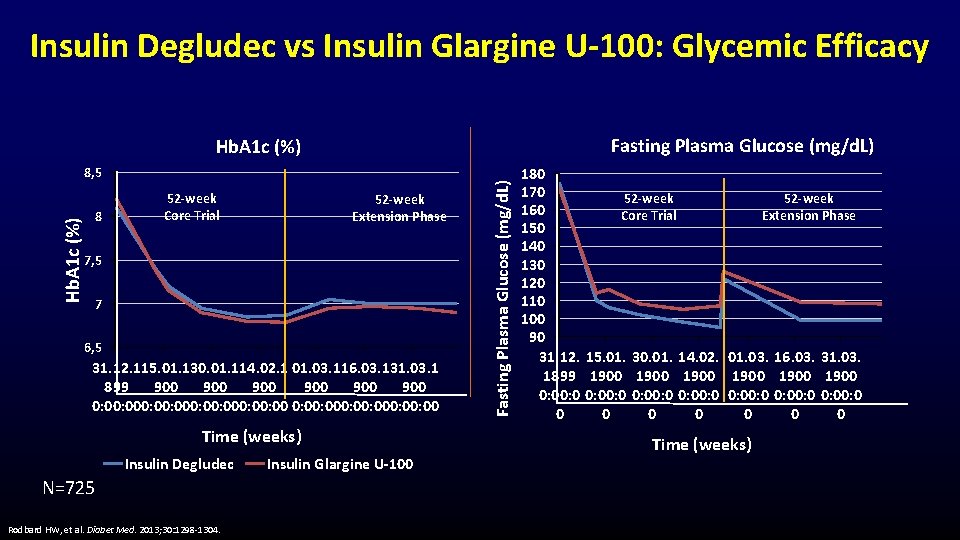
Insulin Degludec vs Insulin Glargine U-100: Glycemic Efficacy Fasting Plasma Glucose (mg/d. L) Hb. A 1 c (%) 8, 5 8 52 -week Core Trial 52 -week Extension Phase 7, 5 7 6, 5 31. 12. 115. 01. 130. 01. 114. 02. 1 01. 03. 116. 03. 131. 03. 1 899 900 900 900 0: 00: 000: 00: 000: 00 Time (weeks) Insulin Degludec N=725 Rodbard HW, et al. Diabet Med. 2013; 30: 1298 -1304. Insulin Glargine U-100 Fasting Plasma Glucose (mg/d. L) Hb. A 1 c (%) 180 170 52 -week 160 Core Trial Extension Phase 150 140 130 120 110 100 90 31. 12. 15. 01. 30. 01. 14. 02. 01. 03. 16. 03. 31. 03. 1899 1900 1900 0: 00: 0 0: 00: 0 0 0 0 0 Time (weeks)
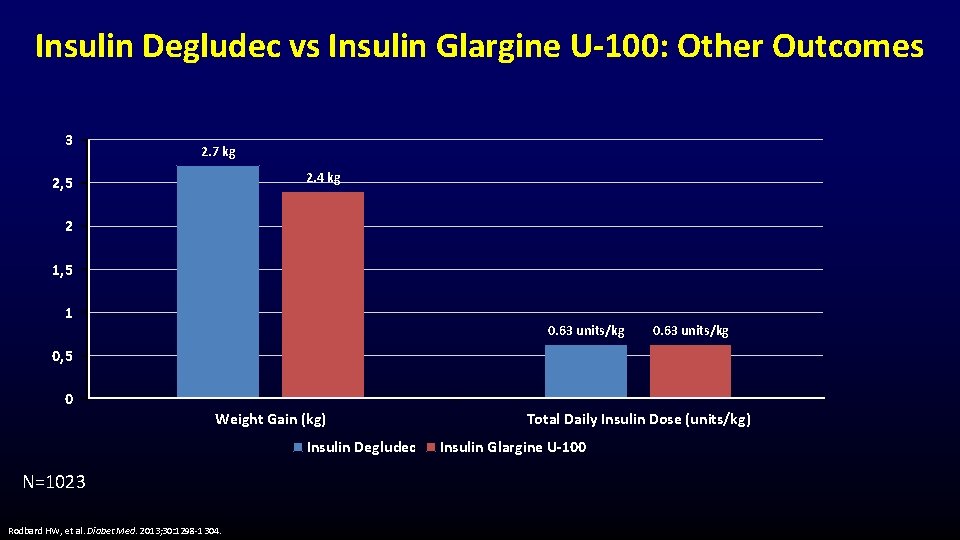
Insulin Degludec vs Insulin Glargine U-100: Other Outcomes 3 2. 7 kg 2. 4 kg 2, 5 2 1, 5 1 0. 63 units/kg 0, 5 0 Weight Gain (kg) Insulin Degludec N=1023 Rodbard HW, et al. Diabet Med. 2013; 30: 1298 -1304. Total Daily Insulin Dose (units/kg) Insulin Glargine U-100
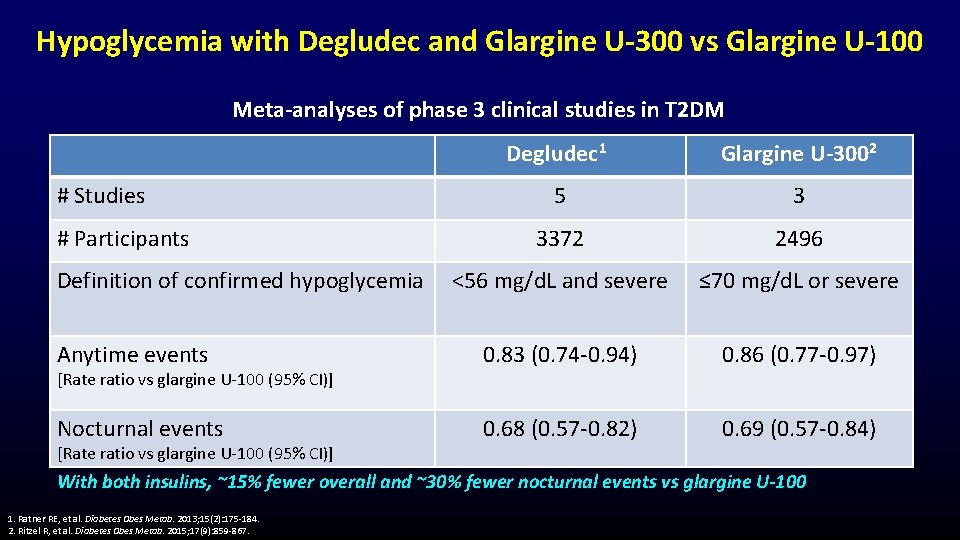
Hypoglycemia with Degludec and Glargine U-300 vs Glargine U-100 Meta-analyses of phase 3 clinical studies in T 2 DM Degludec 1 Glargine U-3002 5 3 3372 2496 <56 mg/d. L and severe ≤ 70 mg/d. L or severe Anytime events 0. 83 (0. 74 -0. 94) 0. 86 (0. 77 -0. 97) Nocturnal events 0. 68 (0. 57 -0. 82) 0. 69 (0. 57 -0. 84) # Studies # Participants Definition of confirmed hypoglycemia [Rate ratio vs glargine U-100 (95% CI)] With both insulins, ~15% fewer overall and ~30% fewer nocturnal events vs glargine U-100 1. Ratner RE, et al. Diabetes Obes Metab. 2013; 15(2): 175 -184. 2. Ritzel R, et al. Diabetes Obes Metab. 2015; 17(9): 859 -867.
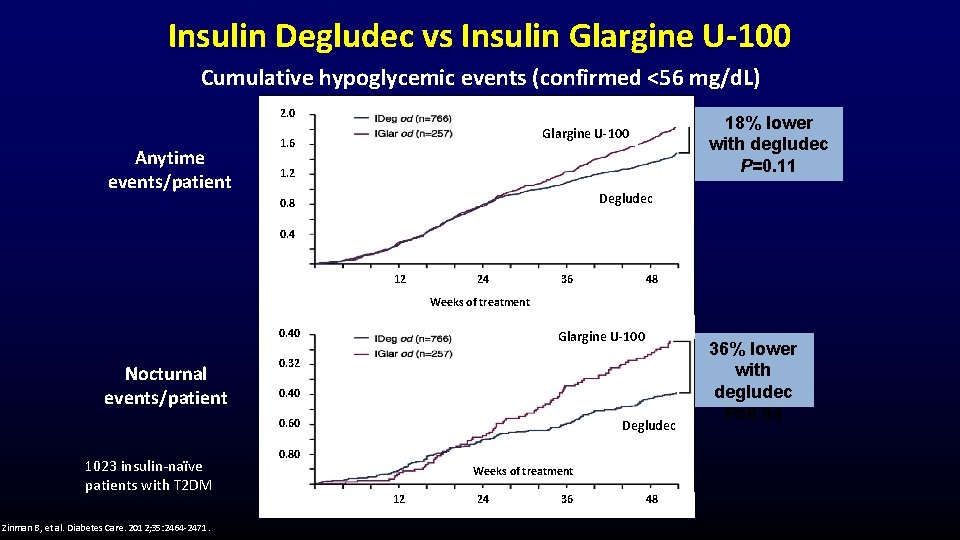
Insulin Degludec vs Insulin Glargine U-100 Cumulative hypoglycemic events (confirmed <56 mg/d. L) 2. 0 Anytime events/patient 18% lower with degludec P=0. 11 Glargine U-100 1. 6 1. 2 Degludec 0. 8 0. 4 12 24 36 48 Weeks of treatment 0. 40 Nocturnal events/patient Glargine U-100 0. 32 0. 40 0. 60 1023 insulin-naïve patients with T 2 DM Zinman B, et al. Diabetes Care. 2012; 35: 2464 -2471. Degludec 0. 80 Weeks of treatment 12 24 36 48 36% lower with degludec P=0. 04
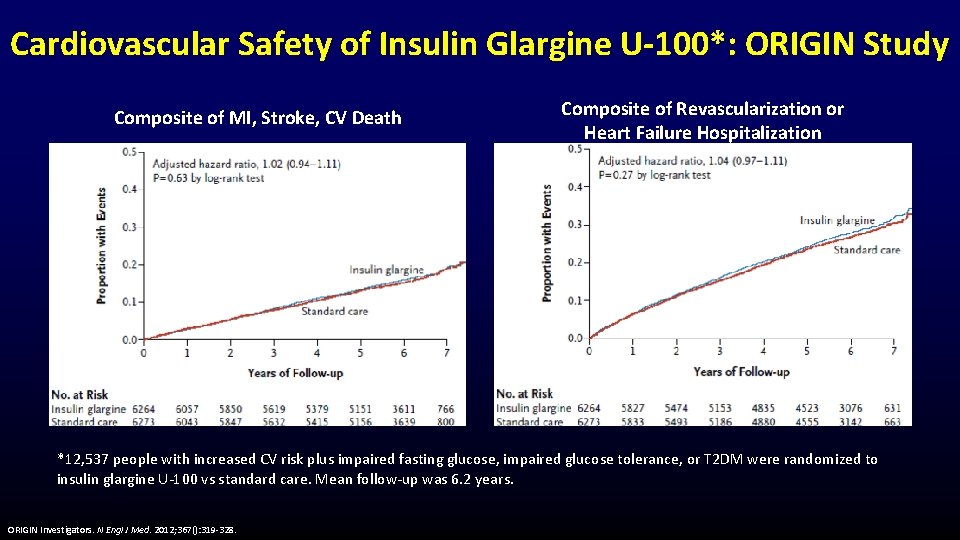
Cardiovascular Safety of Insulin Glargine U-100*: ORIGIN Study Composite of MI, Stroke, CV Death Composite of Revascularization or Heart Failure Hospitalization *12, 537 people with increased CV risk plus impaired fasting glucose, impaired glucose tolerance, or T 2 DM were randomized to insulin glargine U-100 vs standard care. Mean follow-up was 6. 2 years. ORIGIN Investigators. N Engl J Med. 2012; 367(): 319 -328.
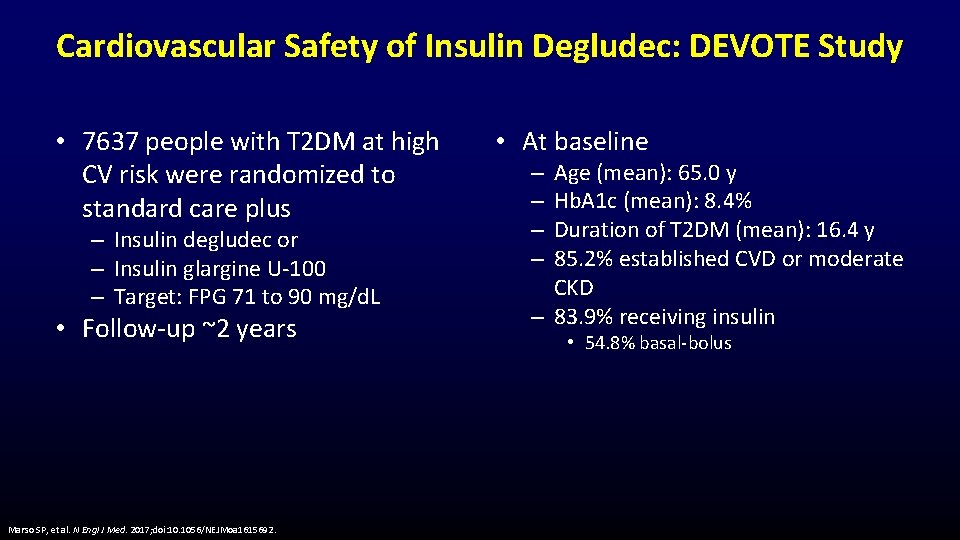
Cardiovascular Safety of Insulin Degludec: DEVOTE Study • 7637 people with T 2 DM at high CV risk were randomized to standard care plus – Insulin degludec or – Insulin glargine U-100 – Target: FPG 71 to 90 mg/d. L • Follow-up ~2 years Marso SP, et al. N Engl J Med. 2017; doi: 10. 1056/NEJMoa 1615692. • At baseline Age (mean): 65. 0 y Hb. A 1 c (mean): 8. 4% Duration of T 2 DM (mean): 16. 4 y 85. 2% established CVD or moderate CKD – 83. 9% receiving insulin – – • 54. 8% basal-bolus
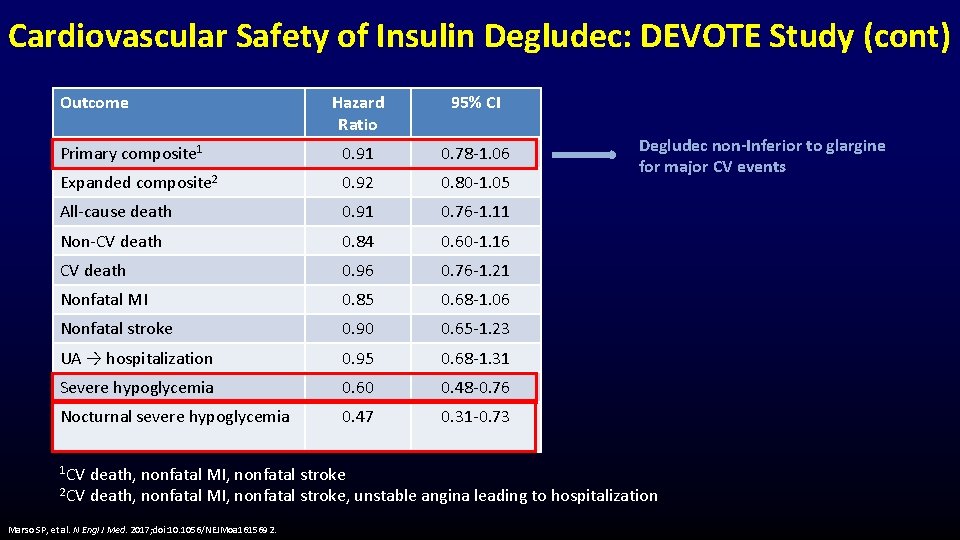
Cardiovascular Safety of Insulin Degludec: DEVOTE Study (cont) Outcome Hazard Ratio 95% CI Primary composite 1 0. 91 0. 78 -1. 06 Expanded composite 2 0. 92 0. 80 -1. 05 All-cause death 0. 91 0. 76 -1. 11 Non-CV death 0. 84 0. 60 -1. 16 CV death 0. 96 0. 76 -1. 21 Nonfatal MI 0. 85 0. 68 -1. 06 Nonfatal stroke 0. 90 0. 65 -1. 23 UA → hospitalization 0. 95 0. 68 -1. 31 Severe hypoglycemia 0. 60 0. 48 -0. 76 Nocturnal severe hypoglycemia 0. 47 0. 31 -0. 73 1 CV Degludec non-Inferior to glargine for major CV events death, nonfatal MI, nonfatal stroke 2 CV death, nonfatal MI, nonfatal stroke, unstable angina leading to hospitalization Marso SP, et al. N Engl J Med. 2017; doi: 10. 1056/NEJMoa 1615692.
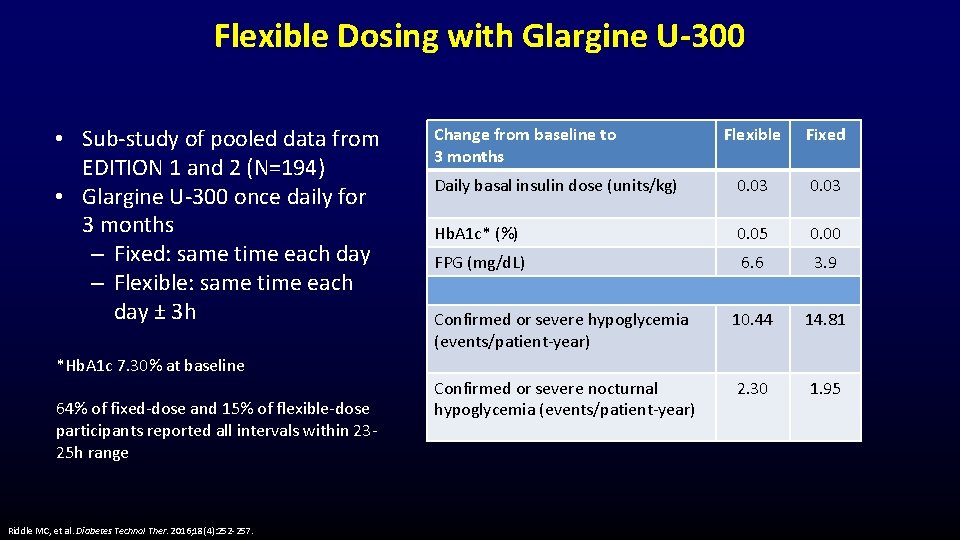
Flexible Dosing with Glargine U-300 • Sub-study of pooled data from EDITION 1 and 2 (N=194) • Glargine U-300 once daily for 3 months – Fixed: same time each day – Flexible: same time each day ± 3 h Change from baseline to 3 months Flexible Fixed Daily basal insulin dose (units/kg) 0. 03 Hb. A 1 c* (%) 0. 05 0. 00 FPG (mg/d. L) 6. 6 3. 9 Confirmed or severe hypoglycemia (events/patient-year) 10. 44 14. 81 Confirmed or severe nocturnal hypoglycemia (events/patient-year) 2. 30 1. 95 *Hb. A 1 c 7. 30% at baseline 64% of fixed-dose and 15% of flexible-dose participants reported all intervals within 2325 h range Riddle MC, et al. Diabetes Technol Ther. 2016; 18(4): 252 -257.
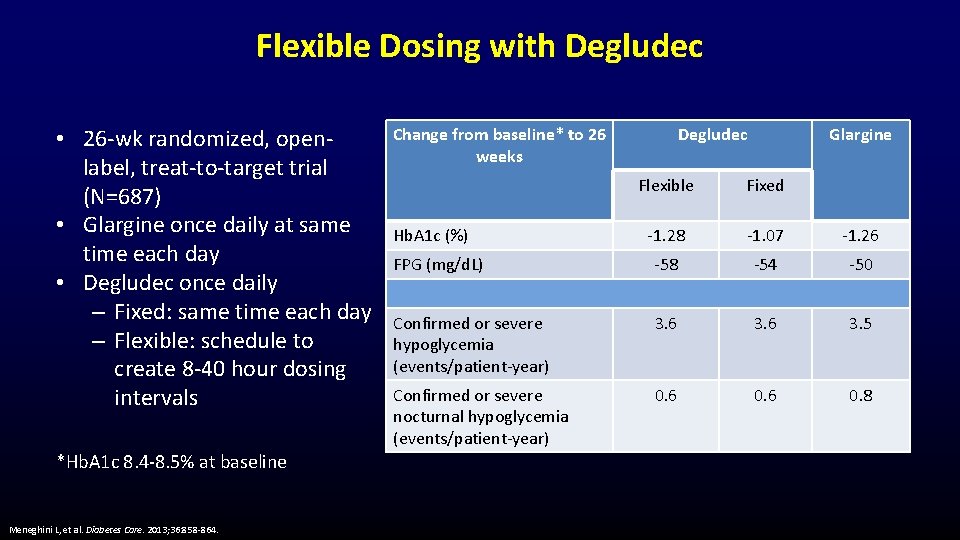
Flexible Dosing with Degludec • 26 -wk randomized, openlabel, treat-to-target trial (N=687) • Glargine once daily at same time each day • Degludec once daily – Fixed: same time each day – Flexible: schedule to create 8 -40 hour dosing intervals *Hb. A 1 c 8. 4 -8. 5% at baseline Meneghini L, et al. Diabetes Care. 2013; 36: 858 -864. Change from baseline* to 26 weeks Degludec Glargine Flexible Fixed -1. 28 -1. 07 -1. 26 FPG (mg/d. L) -58 -54 -50 Confirmed or severe hypoglycemia (events/patient-year) 3. 6 3. 5 Confirmed or severe nocturnal hypoglycemia (events/patient-year) 0. 6 0. 8 Hb. A 1 c (%)

Escalation vs Intensification of Basal Insulin Vivian A. Fonseca, MD, FRCP Professor of Medicine and Pharmacology Tullis Tulane Alumni Chair in Diabetes Chief, Section of Endocrinology Tulane University School of Medicine New Orleans, Louisiana Jonathan D. Leffert, MD, FACP, FACE, ECNU Managing Partner, North Texas Endocrine Center President, American Association of Clinical Endocrinologists Dallas, Texas

Case Scenario: George 56 yo white male with a 7 -y history of T 2 DM Titrates glargine U-100 with a mean FPG 130 -145 mg/d. L Hb. A 1 c 7. 8% SMBG 2 -3 days/week Has occasional night sweats and restless sleep at 2 -3 am Current medications – Metformin 1000 mg bid – Pioglitazone 30 mg q. AM – Glargine U-100 65 units q. HS • Vital signs: 5’ 10”; weight 216 lbs; BMI 31. 0 kg/m 2 • • • What considerations do you have?
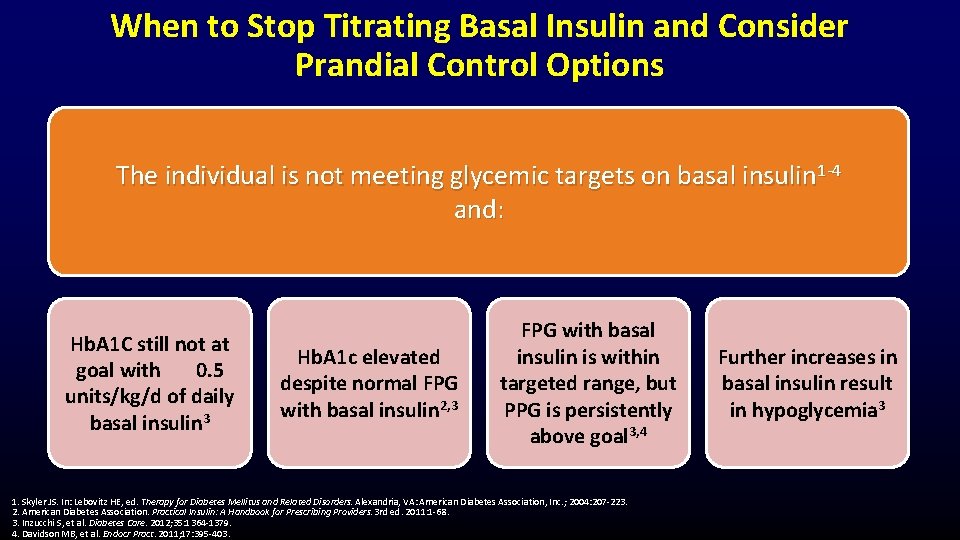
When to Stop Titrating Basal Insulin and Consider Prandial Control Options The individual is not meeting glycemic targets on basal insulin 1 -4 and: Hb. A 1 C still not at goal with 0. 5 units/kg/d of daily basal insulin 3 Hb. A 1 c elevated despite normal FPG with basal insulin 2, 3 FPG with basal insulin is within targeted range, but PPG is persistently above goal 3, 4 1. Skyler JS. In: Lebovitz HE, ed. Therapy for Diabetes Mellitus and Related Disorders. Alexandria, VA: American Diabetes Association, Inc. ; 2004: 207 -223. 2. American Diabetes Association. Practical Insulin: A Handbook for Prescribing Providers. 3 rd ed. 2011: 1 -68. 3. Inzucchi S, et al. Diabetes Care. 2012; 35: 1364 -1379. 4. Davidson MB, et al. Endocr Pract. 2011; 17: 395 -403. Further increases in basal insulin result in hypoglycemia 3
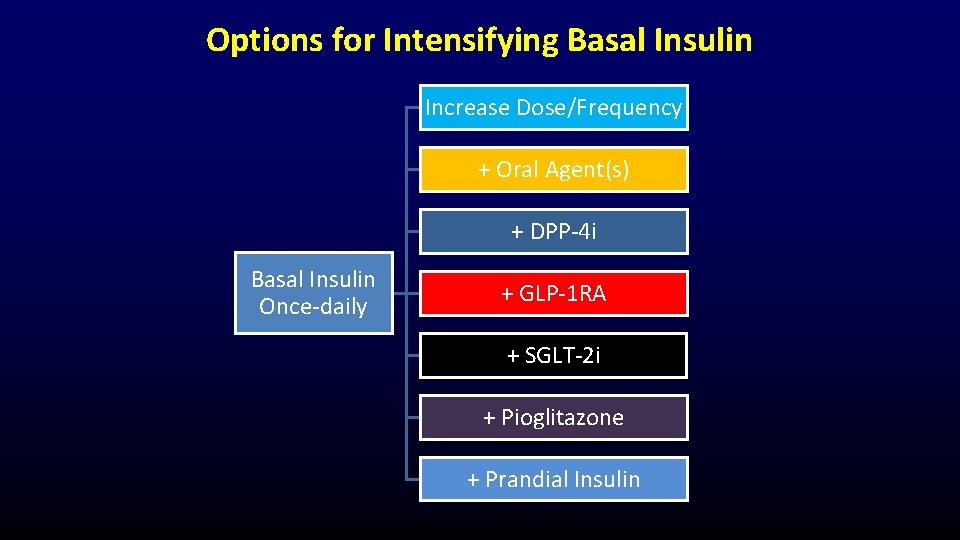
Options for Intensifying Basal Insulin Increase Dose/Frequency + Oral Agent(s) + DPP-4 i Basal Insulin Once-daily + GLP-1 RA + SGLT-2 i + Pioglitazone + Prandial Insulin
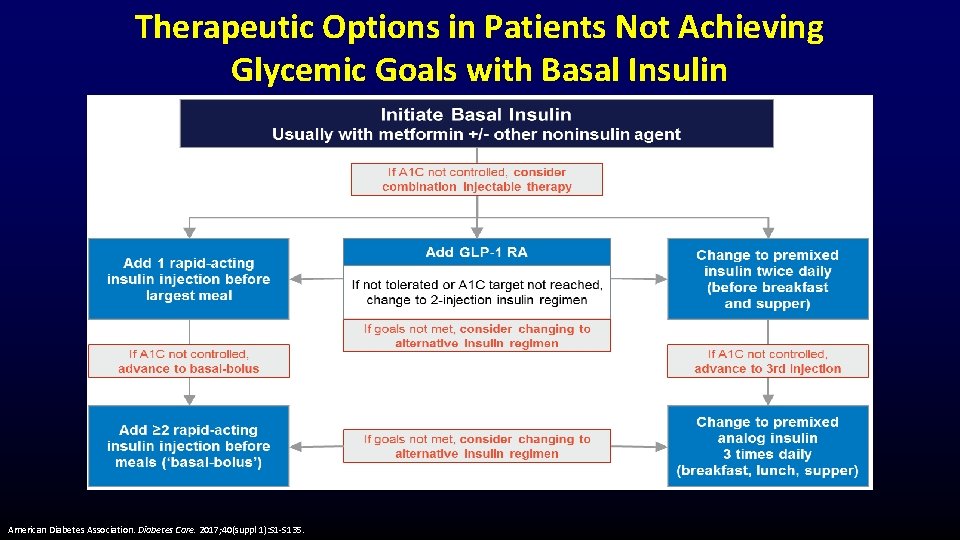
Therapeutic Options in Patients Not Achieving Glycemic Goals with Basal Insulin American Diabetes Association. Diabetes Care. 2017; 40(suppl 1): S 1 -S 135.
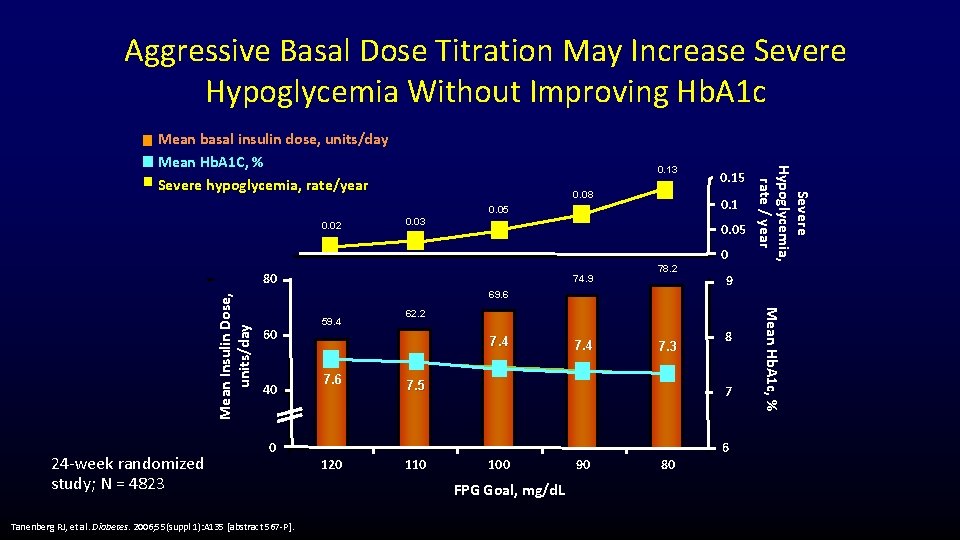
Aggressive Basal Dose Titration May Increase Severe Hypoglycemia Without Improving Hb. A 1 c 0. 13 0. 08 0. 1 0. 05 0. 02 0. 03 0. 05 24 -week randomized study; N = 4823 74. 9 78. 2 69. 6 60 40 0 Tanenberg RJ, et al. Diabetes. 2006; 55(suppl 1): A 135 [abstract 567 -P]. 120 9 62. 2 7. 4 7. 6 0 7. 4 7. 3 7. 5 8 7 6 110 100 FPG Goal, mg/d. L 90 80 Mean Hb. A 1 c, % Mean Insulin Dose, units/day 80 59. 4 0. 15 Severe Hypoglycemia, rate / year Mean basal insulin dose, units/day Mean Hb. A 1 C, % Severe hypoglycemia, rate/year
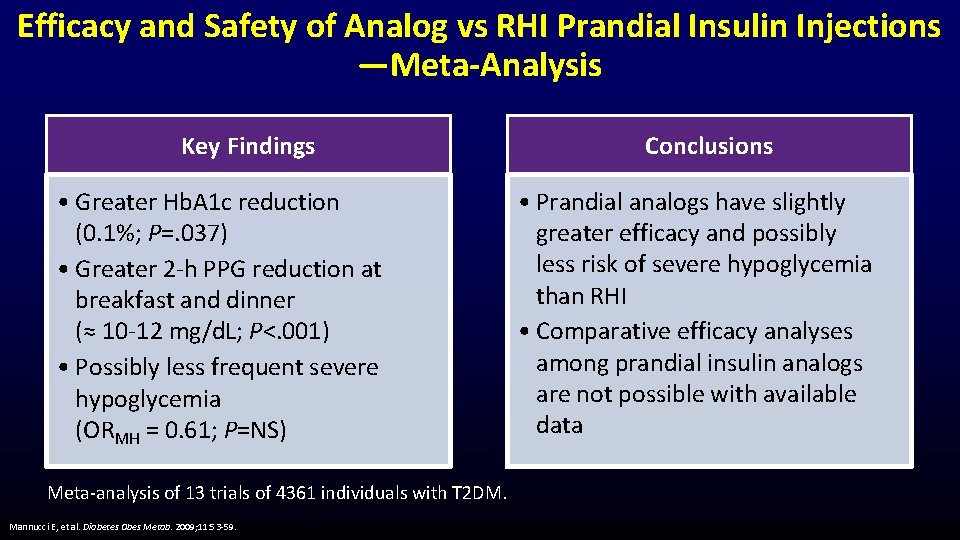
Efficacy and Safety of Analog vs RHI Prandial Insulin Injections —Meta-Analysis Key Findings • Greater Hb. A 1 c reduction (0. 1%; P=. 037) • Greater 2 -h PPG reduction at breakfast and dinner (≈ 10 -12 mg/d. L; P<. 001) • Possibly less frequent severe hypoglycemia (ORMH = 0. 61; P=NS) Meta-analysis of 13 trials of 4361 individuals with T 2 DM. Mannucci E, et al. Diabetes Obes Metab. 2009; 11: 53 -59. Conclusions • Prandial analogs have slightly greater efficacy and possibly less risk of severe hypoglycemia than RHI • Comparative efficacy analyses among prandial insulin analogs are not possible with available data
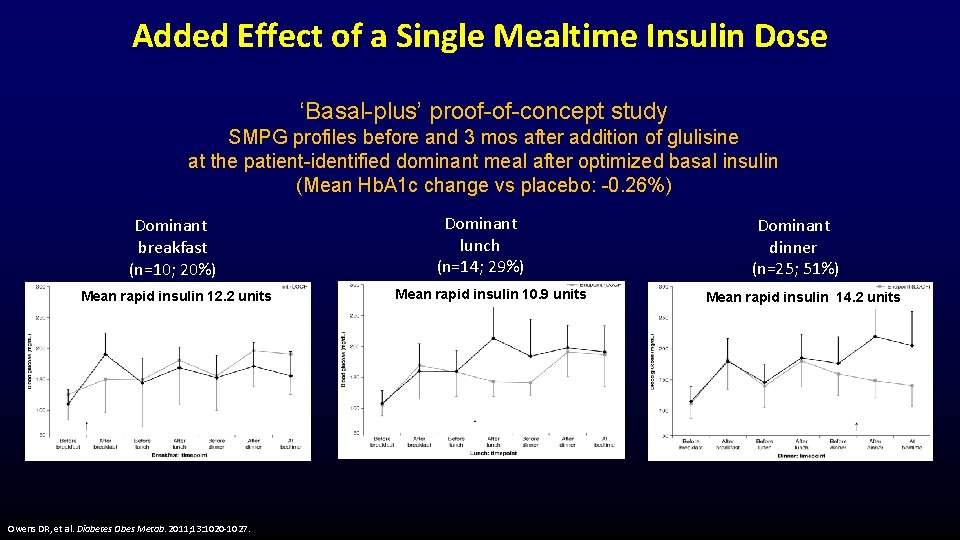
Added Effect of a Single Mealtime Insulin Dose ‘Basal-plus’ proof-of-concept study SMPG profiles before and 3 mos after addition of glulisine at the patient-identified dominant meal after optimized basal insulin (Mean Hb. A 1 c change vs placebo: -0. 26%) Dominant breakfast (n=10; 20%) Mean rapid insulin 12. 2 units Owens DR, et al. Diabetes Obes Metab. 2011; 13: 1020 -1027. Dominant lunch (n=14; 29%) Mean rapid insulin 10. 9 units Dominant dinner (n=25; 51%) Mean rapid insulin 14. 2 units

Basal-Plus Mealtime Insulin • Use rapid-acting analogs (Aspart, Lispro, Glulisine), not RHI – Easier timing, less postprandial hypoglycemia • Start with 1 injection at largest meal: – 4 units and titrate, OR – By weight: 0. 1 unit/kg • Titrate to: – < 140 mg/d. L 2 hours postprandial OR – < 110 mg/d. L next meal or bedtime Garber AJ, et al. Endocr Pract. 2016; 22: 84 -113.
Basal-Plus Mealtime Insulin (cont) • Consider decreasing dose or stopping oral secretagogues • Can continue metformin, TZD, AGI, GLP-1 RA, DPP-4 i • Basal-bolus dosing – ~50% basal insulin and ~50% bolus insulin Garber AJ, et al. Endocr Pract. 2016; 22: 84 -113.
Quality of Life Improves in T 2 DM With Intensification of Insulin Therapy • Multicenter study of 447 patients with insulin-treated T 2 DM and Hb. A 1 c >7% • Patients were transitioned from baseline insulin regimens to basal -bolus using glargine + rapid-acting insulin – Hb. A 1 c declined from 8. 8% to 7. 7% over 6 months (P <. 001) – Nonsevere hypoglycemic episodes decreased • Small but significant improvements with no significant change in hypoglycemia fear – Emotional well-being (P<. 001) – Diabetes symptom distress (P<. 001) – Hypoglycemia fear (P=. 61) Hajos TR, et al. Qual Life Res. 2012; 21: 1359 -1365.

INNOVATIONS IN PRANDIAL INSULINS Vivian A. Fonseca, MD, FRCP Professor of Medicine and Pharmacology Tullis Tulane Alumni Chair in Diabetes Chief, Section of Endocrinology Tulane University School of Medicine New Orleans, Louisiana Jonathan D. Leffert, MD, FACP, FACE, ECNU Managing Partner, North Texas Endocrine Center President, American Association of Clinical Endocrinologists Dallas, Texas
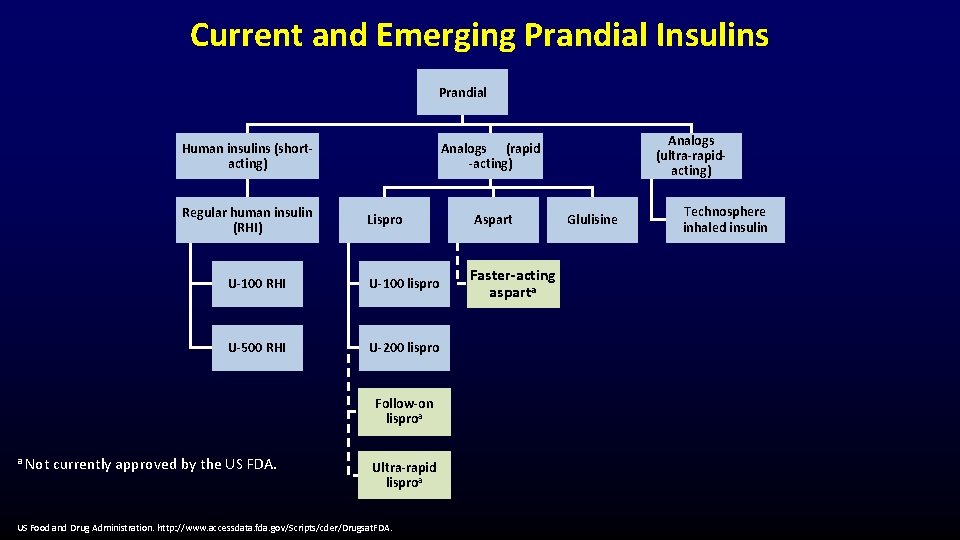
Current and Emerging Prandial Insulins Prandial Human insulins (shortacting) Regular human insulin (RHI) Lispro U-100 RHI U-100 lispro U-500 RHI U-200 lispro Follow-on lisproa a Not currently approved by the US FDA. Analogs (ultra-rapidacting) Analogs (rapid -acting) Ultra-rapid lisproa US Food and Drug Administration. http: //www. accessdata. fda. gov/Scripts/cder/Drugsat. FDA. Aspart Faster-acting asparta Glulisine Technosphere inhaled insulin
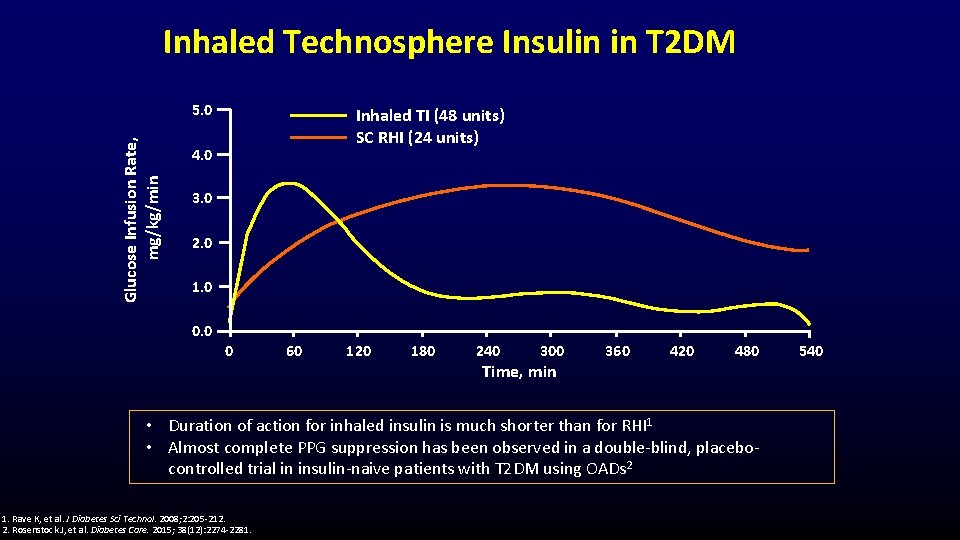
Inhaled Technosphere Insulin in T 2 DM Glucose Infusion Rate, mg/kg/min 5. 0 Inhaled TI (48 units) SC RHI (24 units) 4. 0 3. 0 2. 0 1. 0 0 60 120 180 240 300 360 420 480 Time, min • Duration of action for inhaled insulin is much shorter than for RHI 1 • Almost complete PPG suppression has been observed in a double-blind, placebocontrolled trial in insulin-naive patients with T 2 DM using OADs 2 1. Rave K, et al. J Diabetes Sci Technol. 2008; 2: 205 -212. 2. Rosenstock J, et al. Diabetes Care. 2015; 38(12): 2274 -2281. 540
Inhaled Human Insulin • Limitations of use – Adults – In T 1 DM, use with basal insulin – Not for diabetic ketoacidosis, persons who smoke • Contraindicated in chronic lung disease – Assess lung function prior to and during treatment • Hypokalemia- monitor at-risk persons • Fluid retention/Heart failure with concomitant TZD • Most common adverse events – Hypoglycemia, cough, throat pain/irritation Afrezza [package insert]. Bridgewater, NJ: sanofi-aventis U. S. LLC; January 2016
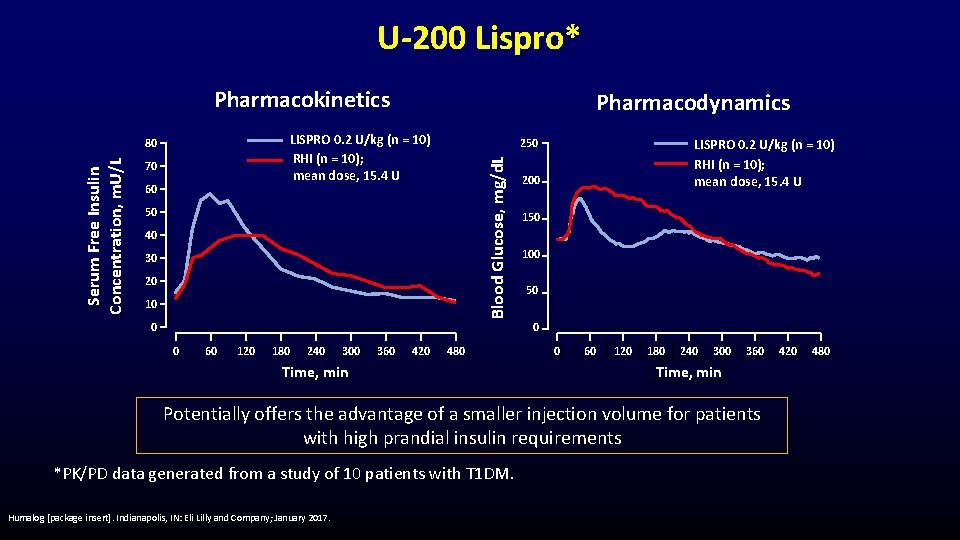
U-200 Lispro* Pharmacokinetics LISPRO 0. 2 U/kg (n = 10) RHI (n = 10); mean dose, 15. 4 U 70 60 250 Blood Glucose, mg/d. L 80 Serum Free Insulin Concentration, m. U/L Pharmacodynamics 50 40 30 20 10 0 LISPRO 0. 2 U/kg (n = 10) RHI (n = 10); mean dose, 15. 4 U 200 150 100 50 0 0 60 120 180 240 300 360 420 480 Time, min 0 60 120 180 240 300 360 Time, min Potentially offers the advantage of a smaller injection volume for patients with high prandial insulin requirements *PK/PD data generated from a study of 10 patients with T 1 DM. Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; January 2017. 420 480

Insulin Lispro U-200 • • Same dose as U-100, but half the volume Hypokalemia- monitor at-risk persons Fluid retention/Heart failure with concomitant TZD Most common adverse events – Hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash Humalog [package insert]. Indianapolis, IN: Eli Lilly and Company; January 2017.
Regular Human Insulin U-500 • Limitations of use – Use in adults/children requiring >200 units insulin/day – Safety/efficacy in combination with other insulins has not been determined • • If using vial/syringe, use only U-500 syringe Hypokalemia- monitor at-risk persons Fluid retention/Heart failure with concomitant TZD Most common adverse events – Hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash Humulin R U-500 [package insert]. Indianapolis, IN: Eli Lilly and Company; March 2017.

PUTTING IT ALL TOGETHER Vivian A. Fonseca, MD, FRCP Professor of Medicine and Pharmacology Tullis Tulane Alumni Chair in Diabetes Chief, Section of Endocrinology Tulane University School of Medicine New Orleans, Louisiana Jonathan D. Leffert, MD, FACP, FACE, ECNU Managing Partner, North Texas Endocrine Center President, American Association of Clinical Endocrinologists Dallas, Texas
Case Scenario: Maria • • • 62 -yo Hispanic female with a 10 -y history of T 2 DM Started glargine U-300 6 months ago as an add-on to orals Titrated glargine; FPG 110 -120 mg/d. L; Hb. A 1 c 7. 5% Works in a busy call center; has a very light breakfast, snack at lunch Admits to being hungry at night; eats largest meal of the day
Case Scenario: Maria (cont) • Current medications – Metformin 1000 mg bid – Glimepiride 4 mg q. AM – Glargine U-300 34 units q. HS • Vital signs: height 5’ 2”; weight 156 lbs; BMI 28. 5 kg/m 2 What are the options for achieving her Hb. A 1 c goal?

Case Scenario: Steven • 42 -yo African-American male with a 3 -y history of T 2 DM • Initially did well on oral agents • Initiated basal insulin due to a relatively rapid rise in blood glucose and worsening glycemic control • Travels frequently for work; demanding and unpredictable work schedule • Has been fatigued and frustrated managing his diabetes • Hb. A 1 c 9. 1%
Case Scenario: Steven (cont) • Current medications – – Metformin 1000 mg bid Glimepiride 2 mg bid Linagliptin 5 mg q. AM Degludec 40 units q. HS • Vital signs: 6’ 0”; weight 184 lbs; BMI 25. 0 kg/m 2 What is your clinical impression?
- Slides: 53