Daily Science Label the following as a molecule

Daily Science Label the following as a molecule, atom, compound, or both (molecule and compound) l H 3 l H 2 O l Ca l Na. Cl l How many and what kind of atoms do you see here: KMn. O 4 l

Chemical Bonds Biology 1
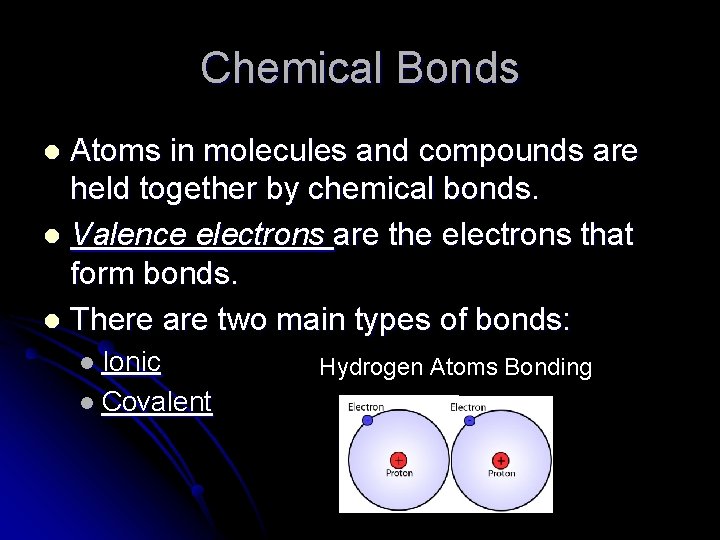
Chemical Bonds Atoms in molecules and compounds are held together by chemical bonds. l Valence electrons are the electrons that form bonds. l There are two main types of bonds: l l Ionic l Covalent Hydrogen Atoms Bonding

Chemical Bonds Ionic Bonds – form when one or more valence electrons are transferred from one atom to another. (Metal + Nonmetal) l Ion – an atom that has a charge because it has either lost or gained electrons. l l If an atom loses electrons, what is its charge? l Positive l If an atom gains electrons, what is its charge? l Negative l Positively charged ions are called cations. Negatively charged ions are called anions.

Fig. 2 -14 -2 Ionic Bonding Na Na Cl Na Sodium atom Cl Chlorine atom Na+ Sodium ion (a cation) Cl– Chloride ion (an anion) Sodium chloride (Na. Cl) Each resulting ion has a completed valence shell

Draw this Staircase!

Metals and Nonmetals Metals are to the left of the staircase on the periodic table l Ex: Iron (Fe), Cobalt (Co), Lithium (Li) l Nonmetals are to the right of the staircase on the periodic table l Ex: Carbon (C), Neon (Ne), Hydrogen (H) l
Chemical Bonds l Covalent Bonds – form when electrons are shared between two atoms. l l Valence electrons travel in the valence shell of both atoms. Electrons must be shared in pairs (one from each atom). l Two electrons Single Covalent Bond l Four electrons Double Covalent Bond l Six electrons Triple Covalent Bond

Fig. 2 -12 Name and Molecular Formula Covalent Bonding in Four Molecules (a) Hydrogen (H 2) (b) Oxygen (O 2) (c) Water (H 2 O) (d) Methane (CH 4) Electron. Lewis Dot Spacedistribution Structure and filling Model Diagram Structural Formula

Ionic and Covalent Bonds Video Copyright © 2008 Pearson Education, Inc. , publishing as Benjamin Cummings

Chemical Bonds l Why do bonds form? l Atoms want to have full valence shells. l. Having a full valence shell makes an atom inert (unreactive). l Atoms will lose/gain/share electrons until their valence shell is full. l The closer a valence shell is to being full, the more reactive an atom is. l Check out this video!
- Slides: 11