Daily Assessment November 29 What is the function

Daily Assessment November 29 What is the function of an enzyme? HL additional question: Describe end product inhibition.
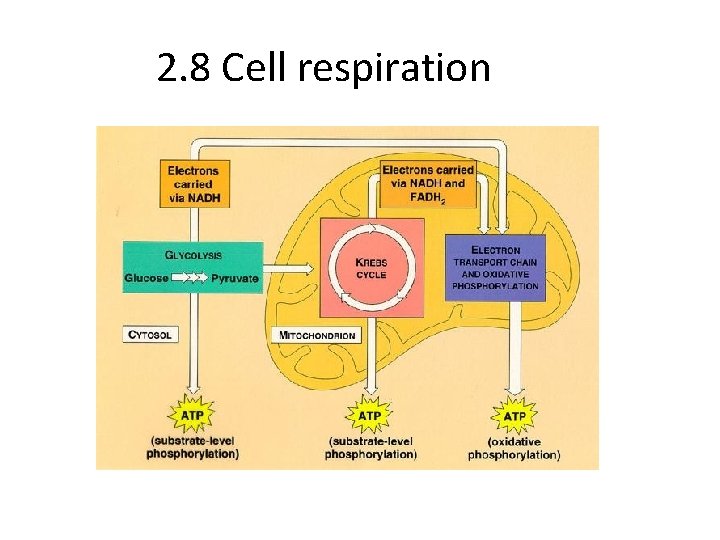
2. 8 Cell respiration
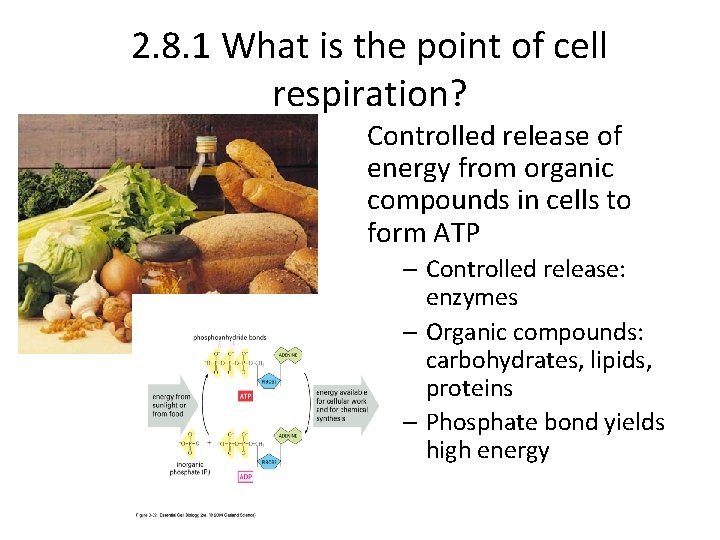
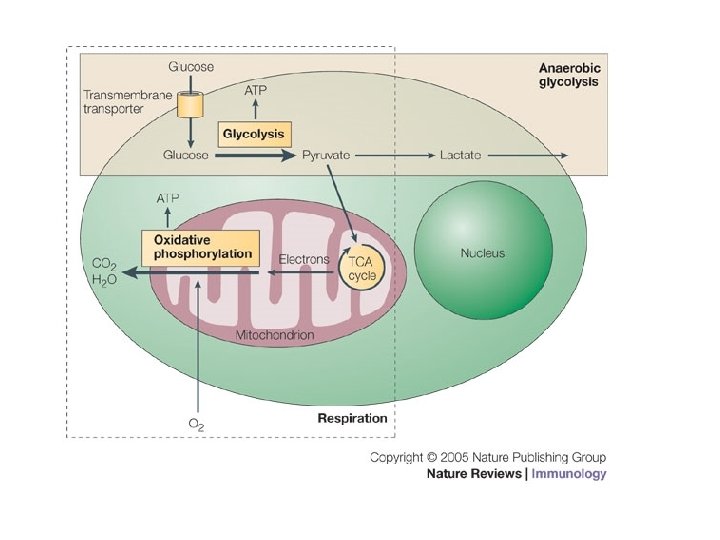
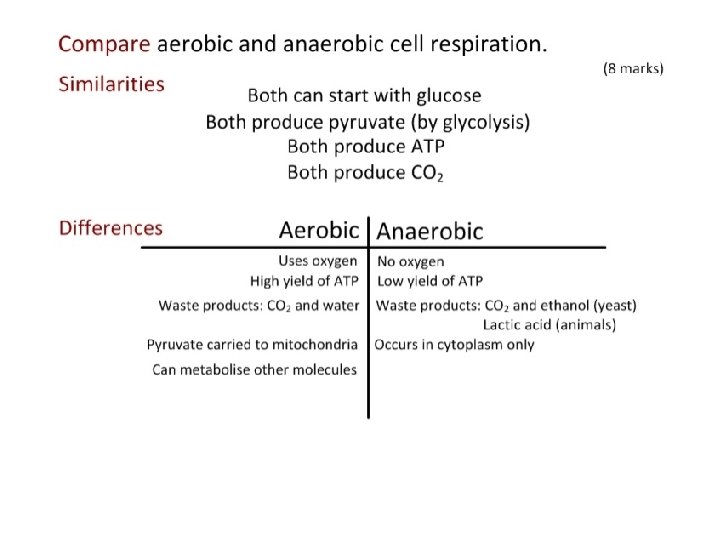
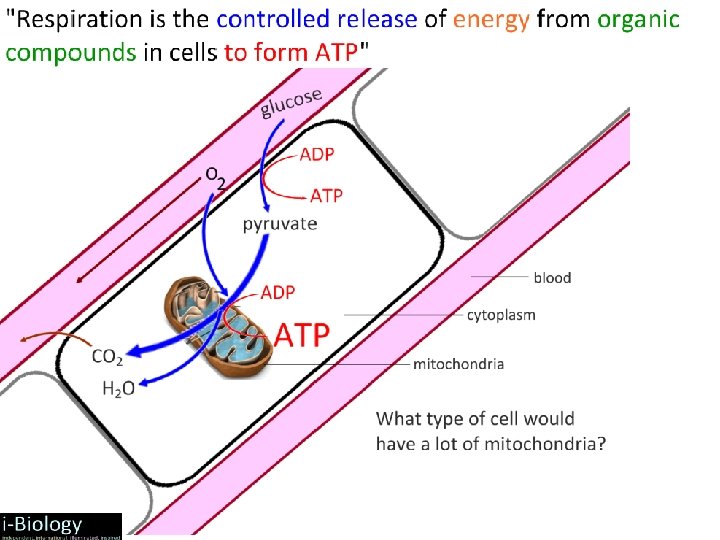
2. 8. 1 What is the point of cell respiration? Controlled release of energy from organic compounds in cells to form ATP – Controlled release: enzymes – Organic compounds: carbohydrates, lipids, proteins – Phosphate bond yields high energy
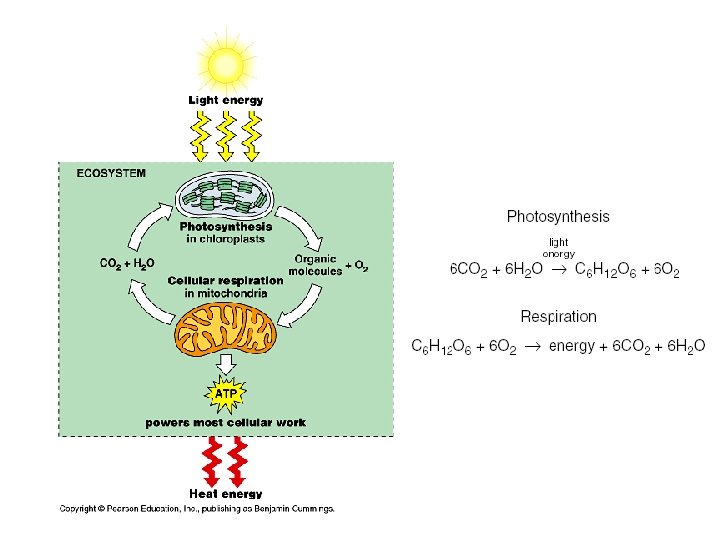
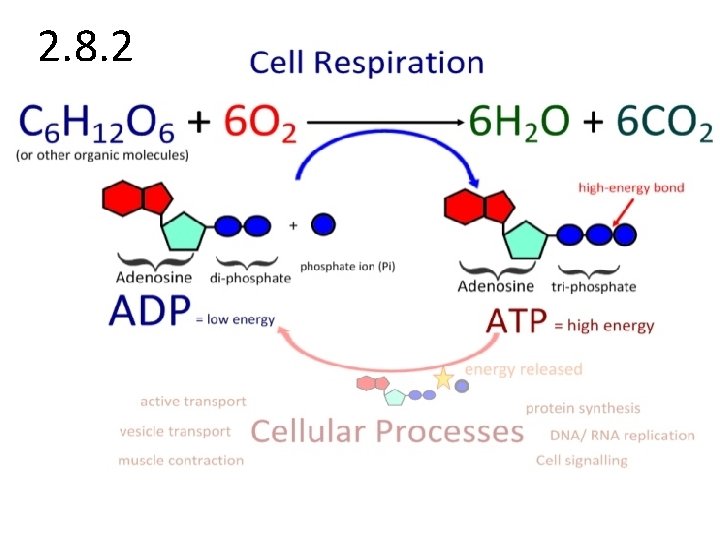
2. 8. 2
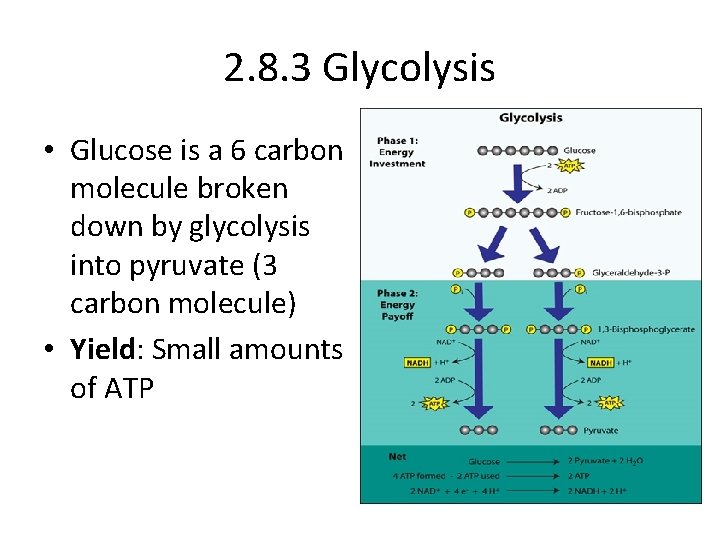
2. 8. 3 Glycolysis • Glucose is a 6 carbon molecule broken down by glycolysis into pyruvate (3 carbon molecule) • Yield: Small amounts of ATP
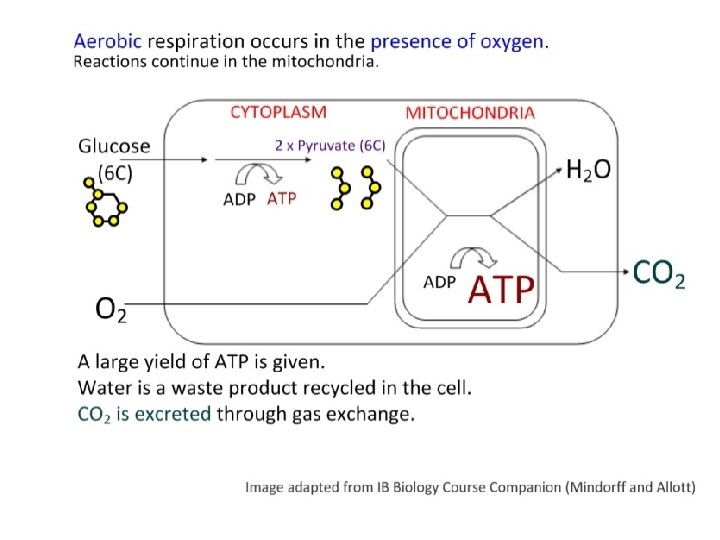
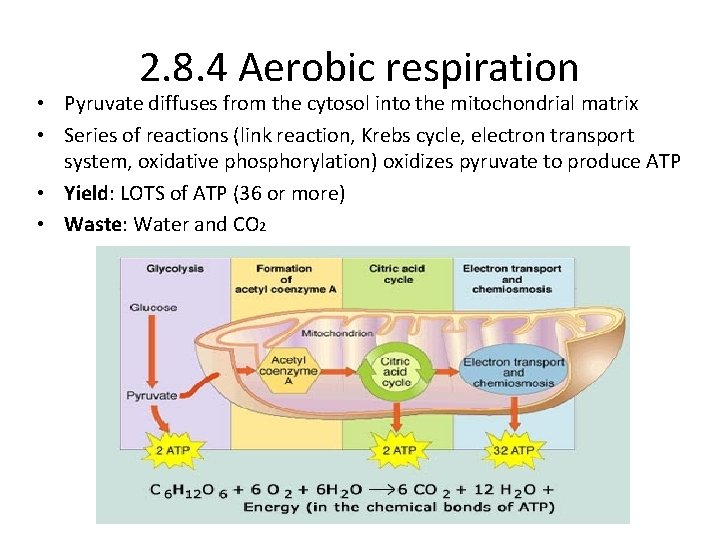
2. 8. 4 Aerobic respiration • Pyruvate diffuses from the cytosol into the mitochondrial matrix • Series of reactions (link reaction, Krebs cycle, electron transport system, oxidative phosphorylation) oxidizes pyruvate to produce ATP • Yield: LOTS of ATP (36 or more) • Waste: Water and CO 2

2. 8. A 1 Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking. Bread is made by adding water to flour, kneading the mixture to make dough and then baking it. Usually an ingredient is added to the dough to create bubbles of gas, so that the baked bread has a lighter texture (e. g. yeast). After kneading (mixing) the dough is kept warm to encourage the yeast to respire. Yeast can respire aerobically or anaerobically, but oxygen in the dough is soon used up so the yeast is forced to respire anaerobically. The carbon dioxide produced by anaerobic cell respiration cannot escape from the dough and forms bubbles causing the dough to swell and rise. Ethanol is also produced by anaerobic cell respiration, but it evaporates during baking. http: //commons. wikimedia. org/wiki/File: Frenchbread 3000 ppx. jpg
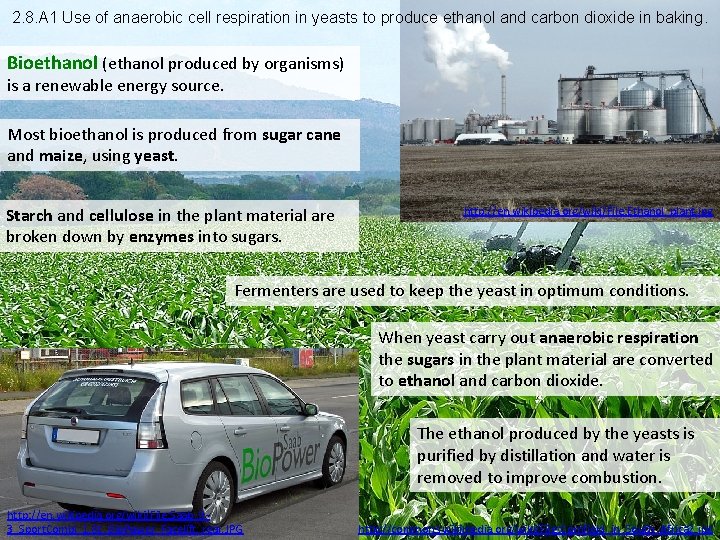
2. 8. A 1 Use of anaerobic cell respiration in yeasts to produce ethanol and carbon dioxide in baking. Bioethanol (ethanol produced by organisms) is a renewable energy source. Most bioethanol is produced from sugar cane and maize, using yeast. Starch and cellulose in the plant material are broken down by enzymes into sugars. http: //en. wikipedia. org/wiki/File: Ethanol_plant. jpg Fermenters are used to keep the yeast in optimum conditions. When yeast carry out anaerobic respiration the sugars in the plant material are converted to ethanol and carbon dioxide. The ethanol produced by the yeasts is purified by distillation and water is removed to improve combustion. http: //en. wikipedia. org/wiki/File: Saab_93_Sport. Combi_1. 8 t_Bio. Power_Facelift_rear. JPG http: //commons. wikimedia. org/wiki/File: Cornfield_in_South_Africa 2. jpg

2. 8. A 2 Lactate production in humans when anaerobic respiration is used to maximize the power of muscle contractions. Certain human activities require anaerobic respiration such as weightlifting and sprinting. Aerobic respiration generates a much greater yield of ATP, but anaerobic respiration can supply ATP very rapidly, as oxygen is not required. Rapid generation of ATP enables us to maximise the power of muscle contractions. Anaerobic cell respiration produces lactate. There is a limit to the concentration that the body can tolerate and this limits how much or how long anaerobic respiration can be done for. Afterwards lactate must be broken down. This involves the use of oxygen. It can take several minutes for enough oxygen to be absorbed for all lactate to be broken down. The demand for oxygen that builds up during a period of anaerobic respiration is called the oxygen debt. http: //commons. wikimedia. org/wiki/File: Osaka 07_D 2 A_Torri_Edwards. jpg

Today • How can we measure respiration? – Lab Bench: Respirometer virtual experiment – http: //www. phschool. com/science/biology_place /labbench/lab 5/concepts. html • Learning Portfolio
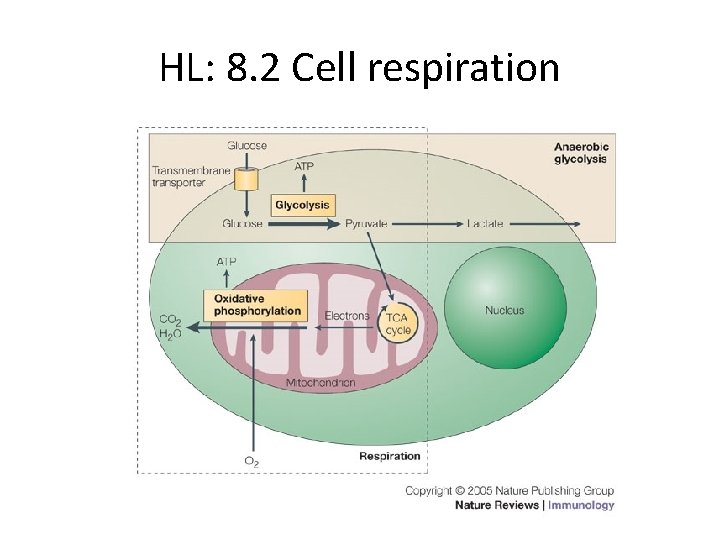
HL: 8. 2 Cell respiration
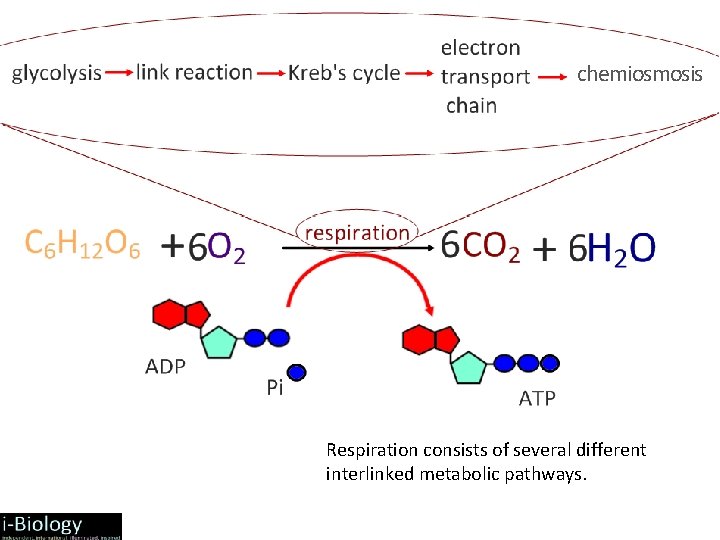
chemiosmosis Respiration consists of several different interlinked metabolic pathways.
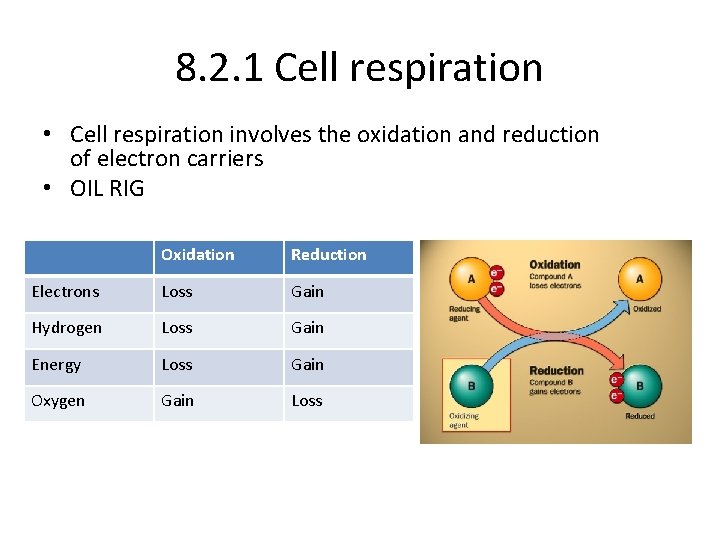
8. 2. 1 Cell respiration • Cell respiration involves the oxidation and reduction of electron carriers • OIL RIG Oxidation Reduction Electrons Loss Gain Hydrogen Loss Gain Energy Loss Gain Oxygen Gain Loss
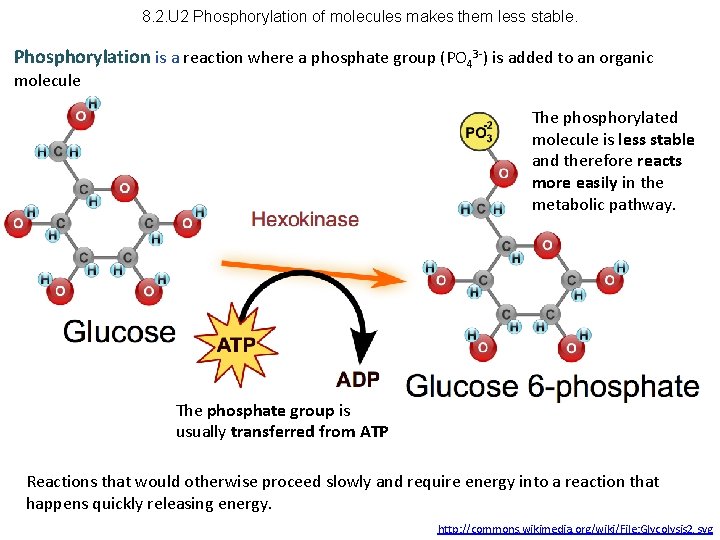
8. 2. U 2 Phosphorylation of molecules makes them less stable. Phosphorylation is a reaction where a phosphate group (PO 43 -) is added to an organic molecule The phosphorylated molecule is less stable and therefore reacts more easily in the metabolic pathway. The phosphate group is usually transferred from ATP Reactions that would otherwise proceed slowly and require energy into a reaction that happens quickly releasing energy. http: //commons. wikimedia. org/wiki/File: Glycolysis 2. svg
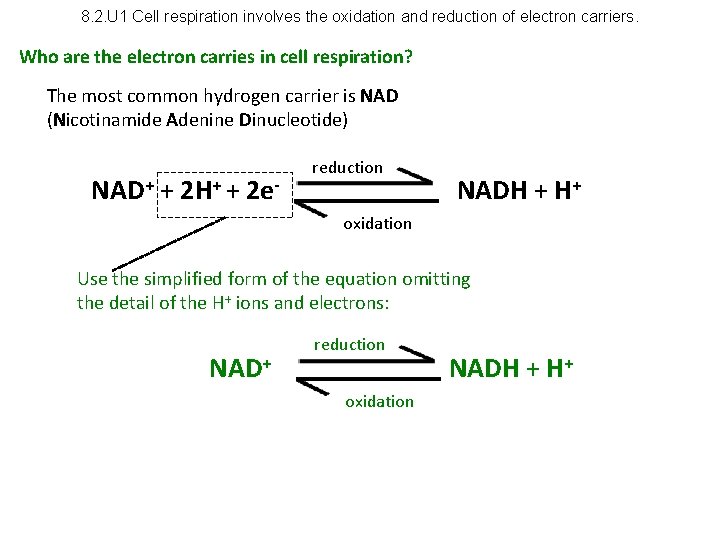
8. 2. U 1 Cell respiration involves the oxidation and reduction of electron carriers. Who are the electron carries in cell respiration? The most common hydrogen carrier is NAD (Nicotinamide Adenine Dinucleotide) NAD+ + 2 H+ + 2 e- reduction NADH + H+ oxidation Use the simplified form of the equation omitting the detail of the H+ ions and electrons: NAD+ reduction oxidation NADH + H+
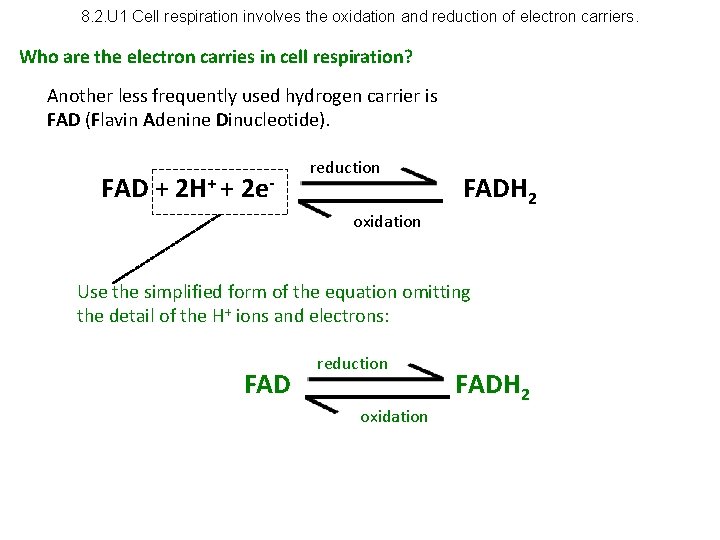
8. 2. U 1 Cell respiration involves the oxidation and reduction of electron carriers. Who are the electron carries in cell respiration? Another less frequently used hydrogen carrier is FAD (Flavin Adenine Dinucleotide). FAD + 2 H+ + 2 e- reduction FADH 2 oxidation Use the simplified form of the equation omitting the detail of the H+ ions and electrons: FAD reduction oxidation FADH 2
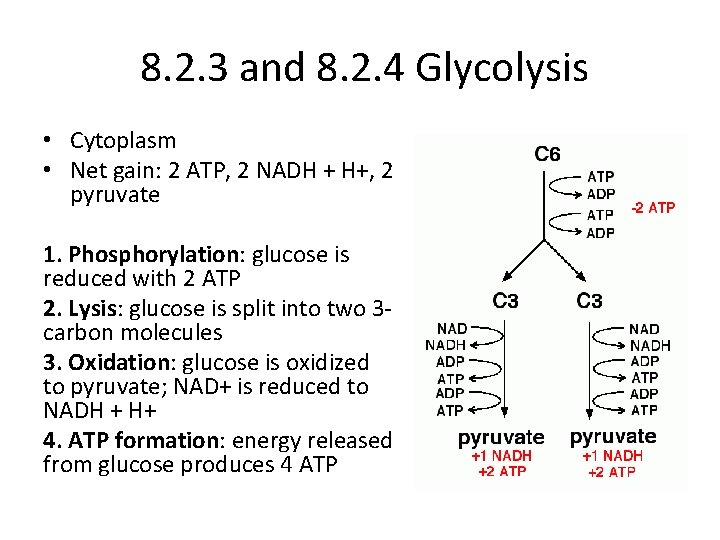
8. 2. 3 and 8. 2. 4 Glycolysis • Cytoplasm • Net gain: 2 ATP, 2 NADH + H+, 2 pyruvate 1. Phosphorylation: glucose is reduced with 2 ATP 2. Lysis: glucose is split into two 3 carbon molecules 3. Oxidation: glucose is oxidized to pyruvate; NAD+ is reduced to NADH + H+ 4. ATP formation: energy released from glucose produces 4 ATP
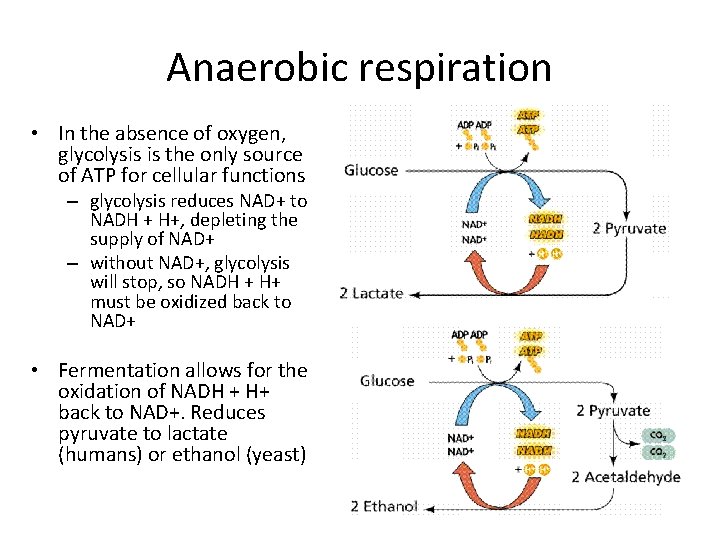
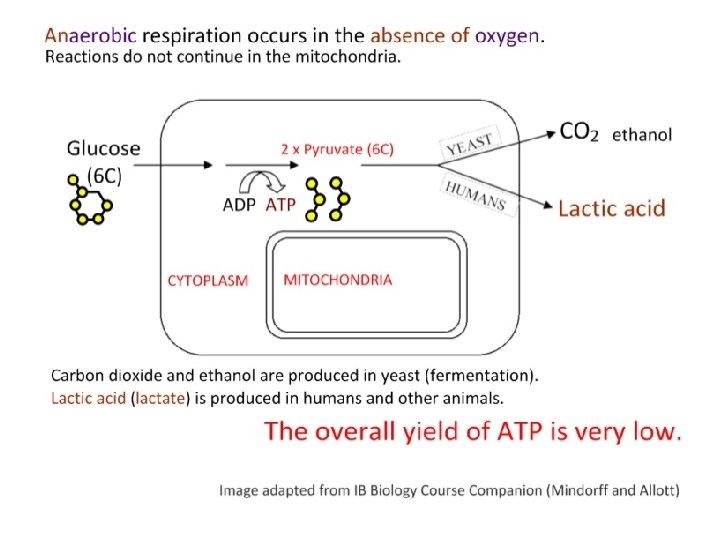
Anaerobic respiration • In the absence of oxygen, glycolysis is the only source of ATP for cellular functions – glycolysis reduces NAD+ to NADH + H+, depleting the supply of NAD+ – without NAD+, glycolysis will stop, so NADH + H+ must be oxidized back to NAD+ • Fermentation allows for the oxidation of NADH + H+ back to NAD+. Reduces pyruvate to lactate (humans) or ethanol (yeast)
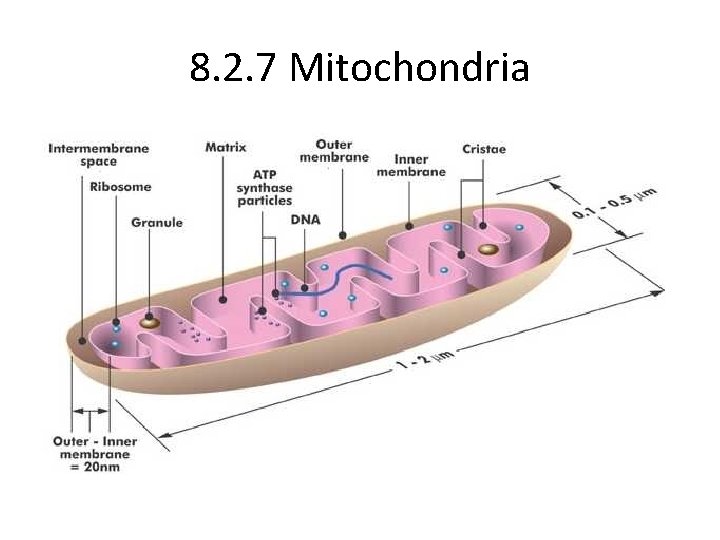
8. 2. 7 Mitochondria
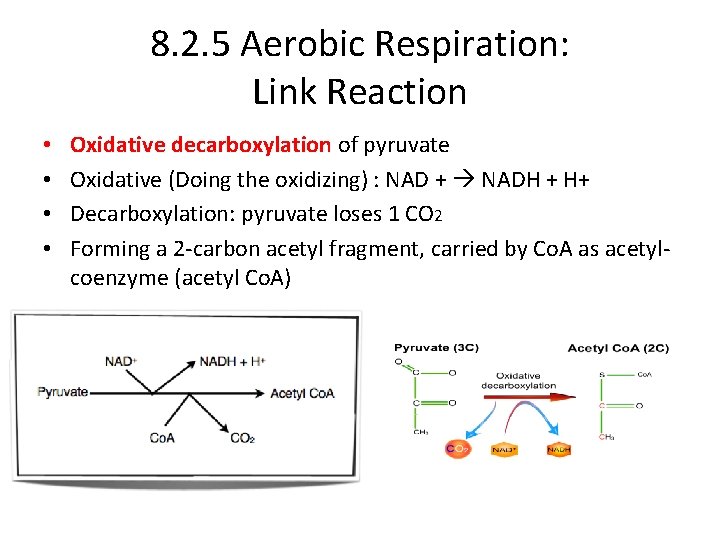
8. 2. 5 Aerobic Respiration: Link Reaction • • Oxidative decarboxylation of pyruvate Oxidative (Doing the oxidizing) : NAD + NADH + H+ Decarboxylation: pyruvate loses 1 CO 2 Forming a 2 -carbon acetyl fragment, carried by Co. A as acetylcoenzyme (acetyl Co. A)
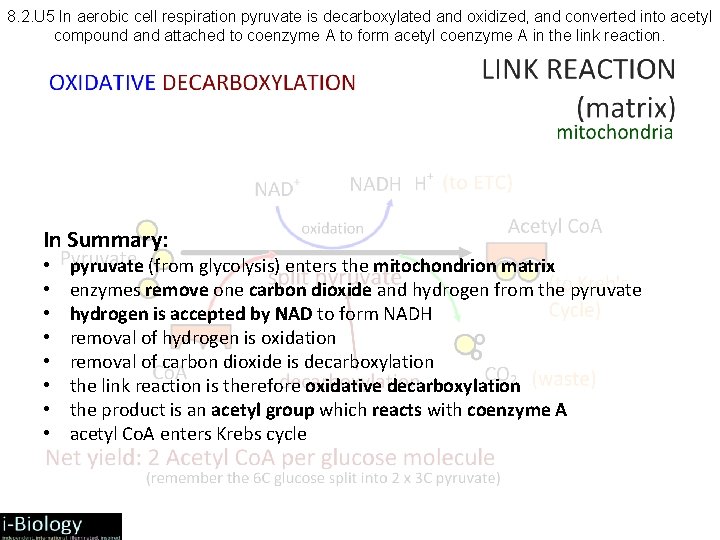
8. 2. U 5 In aerobic cell respiration pyruvate is decarboxylated and oxidized, and converted into acetyl compound attached to coenzyme A to form acetyl coenzyme A in the link reaction. In Summary: • • pyruvate (from glycolysis) enters the mitochondrion matrix enzymes remove one carbon dioxide and hydrogen from the pyruvate hydrogen is accepted by NAD to form NADH removal of hydrogen is oxidation removal of carbon dioxide is decarboxylation the link reaction is therefore oxidative decarboxylation the product is an acetyl group which reacts with coenzyme A acetyl Co. A enters Krebs cycle
hould s u o y t u b , d e ot ne The details are n used in e b n a c s id c a y t t be aware that fa n. aerobic respiratio Glycolysis is not needed, but the reaction is slower.
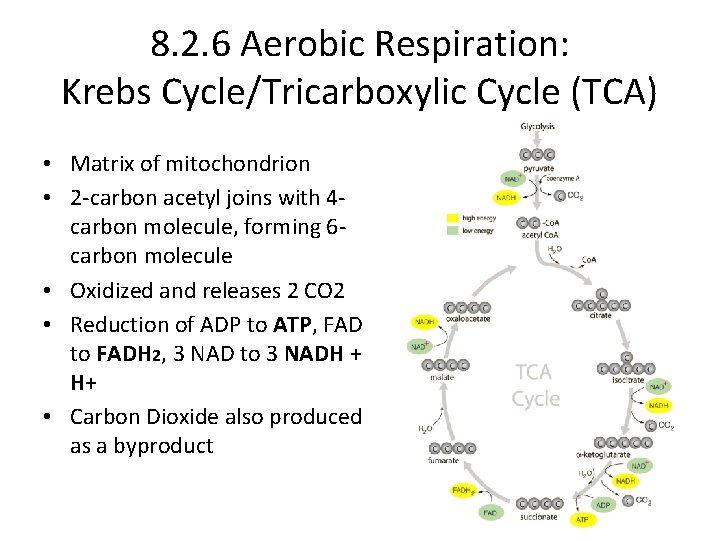
8. 2. 6 Aerobic Respiration: Krebs Cycle/Tricarboxylic Cycle (TCA) • Matrix of mitochondrion • 2 -carbon acetyl joins with 4 carbon molecule, forming 6 carbon molecule • Oxidized and releases 2 CO 2 • Reduction of ADP to ATP, FAD to FADH 2, 3 NAD to 3 NADH + H+ • Carbon Dioxide also produced as a byproduct
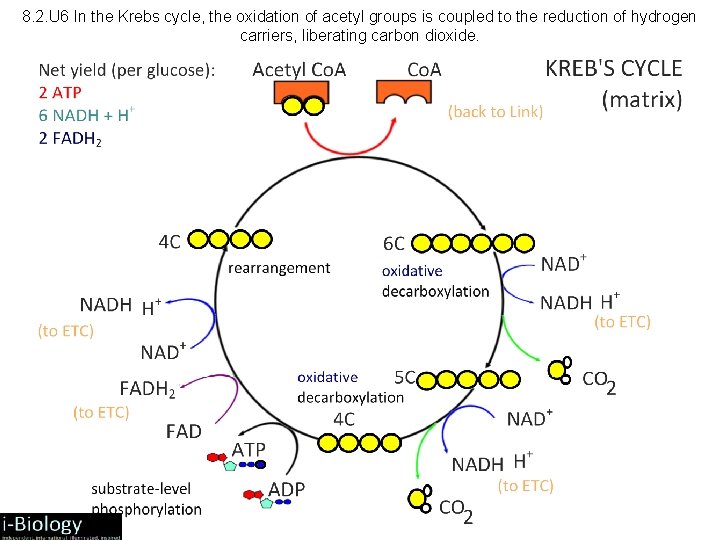
8. 2. U 6 In the Krebs cycle, the oxidation of acetyl groups is coupled to the reduction of hydrogen carriers, liberating carbon dioxide.
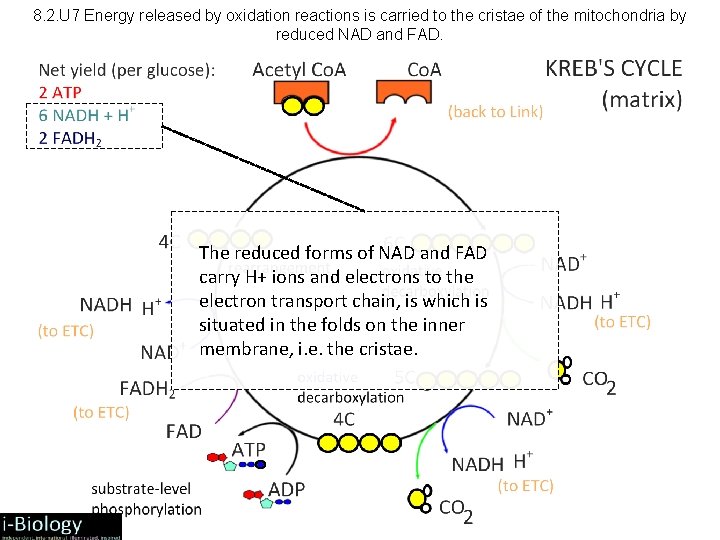
8. 2. U 7 Energy released by oxidation reactions is carried to the cristae of the mitochondria by reduced NAD and FAD. The reduced forms of NAD and FAD carry H+ ions and electrons to the electron transport chain, is which is situated in the folds on the inner membrane, i. e. the cristae.
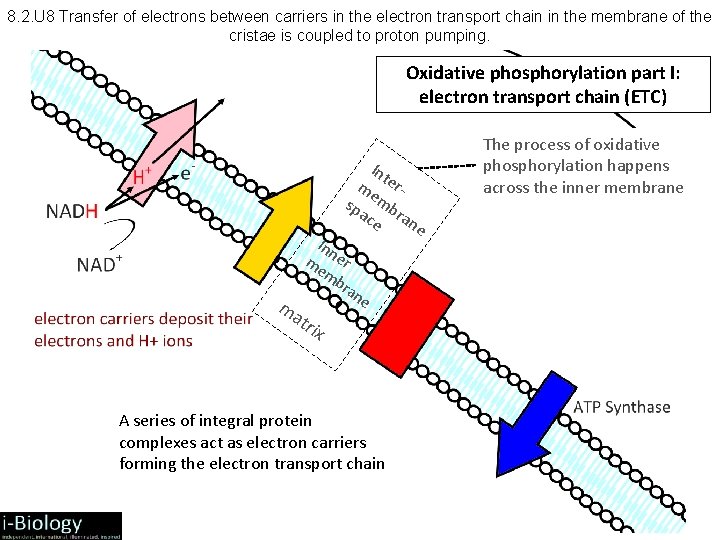
8. 2. U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping. Oxidative phosphorylation part I: electron transport chain (ETC) Int me ersp mb ac ra ne e Inn me er mb ran e m atr ix A series of integral protein complexes act as electron carriers forming the electron transport chain The process of oxidative phosphorylation happens across the inner membrane
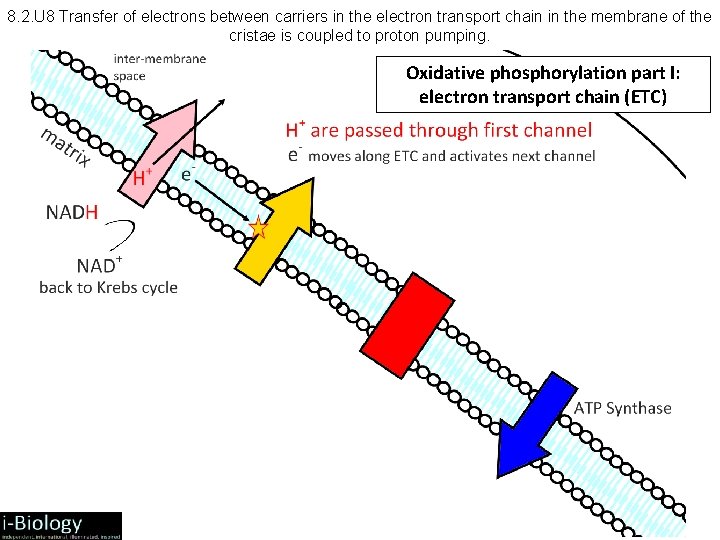
8. 2. U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping. Oxidative phosphorylation part I: electron transport chain (ETC)
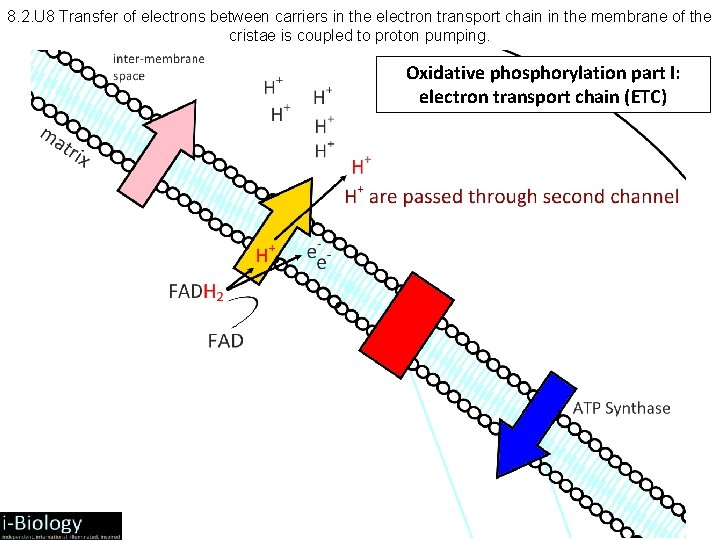
8. 2. U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping. Oxidative phosphorylation part I: electron transport chain (ETC)
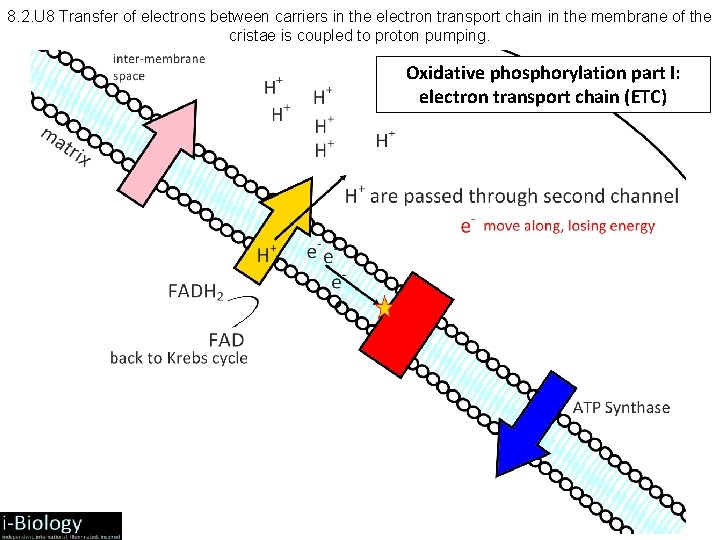
8. 2. U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping. Oxidative phosphorylation part I: electron transport chain (ETC)
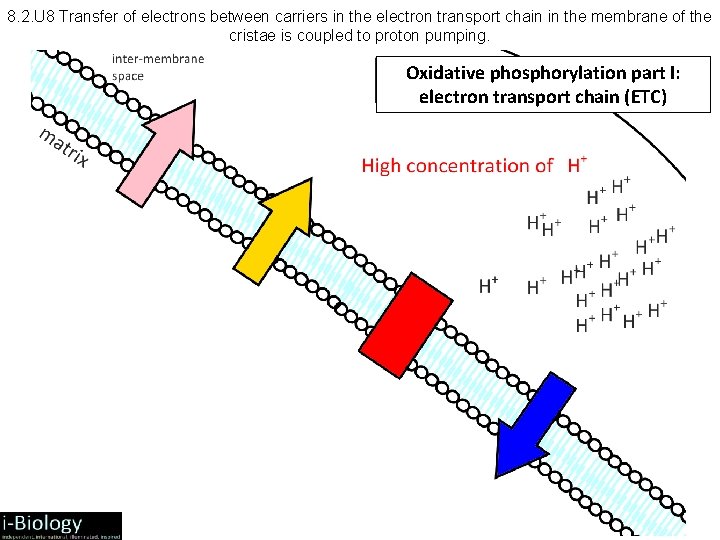
8. 2. U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping. Oxidative phosphorylation part I: electron transport chain (ETC)
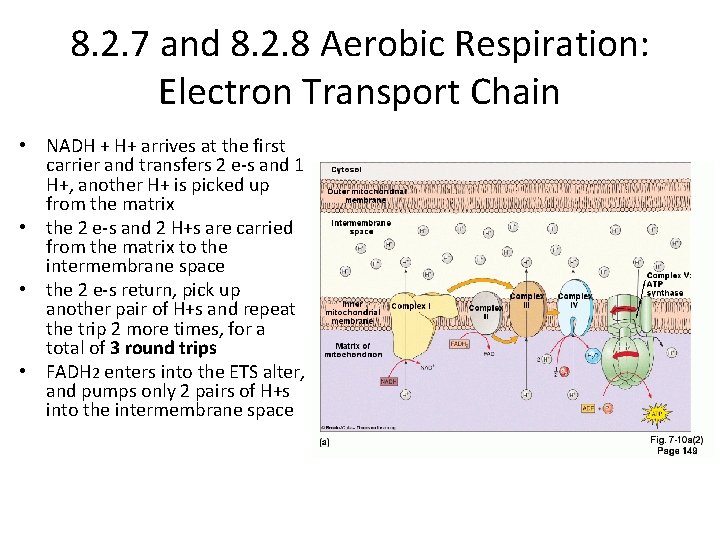
8. 2. 7 and 8. 2. 8 Aerobic Respiration: Electron Transport Chain • NADH + H+ arrives at the first carrier and transfers 2 e-s and 1 H+, another H+ is picked up from the matrix • the 2 e-s and 2 H+s are carried from the matrix to the intermembrane space • the 2 e-s return, pick up another pair of H+s and repeat the trip 2 more times, for a total of 3 round trips • FADH 2 enters into the ETS alter, and pumps only 2 pairs of H+s into the intermembrane space
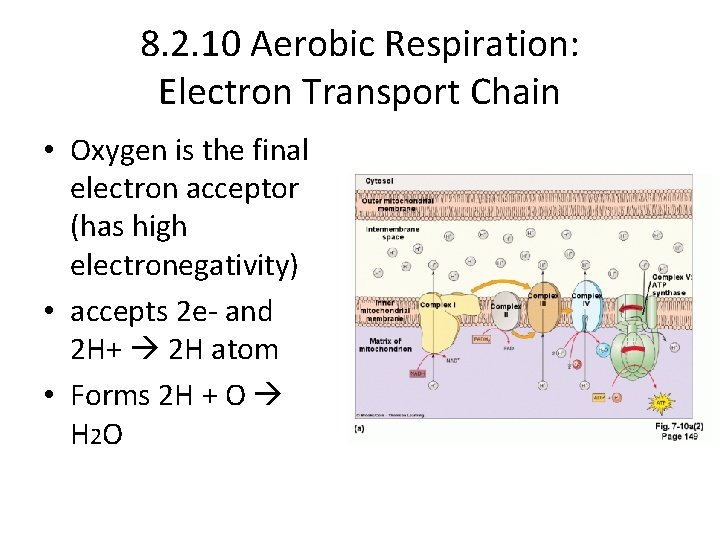
8. 2. 10 Aerobic Respiration: Electron Transport Chain • Oxygen is the final electron acceptor (has high electronegativity) • accepts 2 e- and 2 H+ 2 H atom • Forms 2 H + O H 2 O
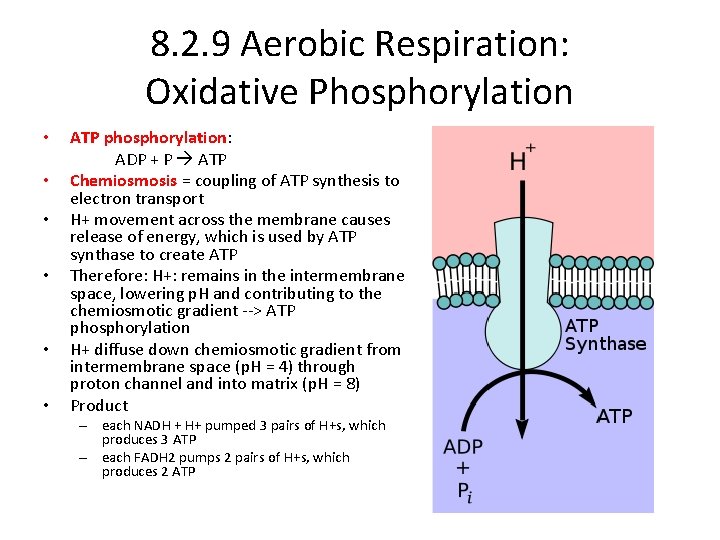
8. 2. 9 Aerobic Respiration: Oxidative Phosphorylation • • • ATP phosphorylation: ADP + P ATP Chemiosmosis = coupling of ATP synthesis to electron transport H+ movement across the membrane causes release of energy, which is used by ATP synthase to create ATP Therefore: H+: remains in the intermembrane space, lowering p. H and contributing to the chemiosmotic gradient --> ATP phosphorylation H+ diffuse down chemiosmotic gradient from intermembrane space (p. H = 4) through proton channel and into matrix (p. H = 8) Product – each NADH + H+ pumped 3 pairs of H+s, which produces 3 ATP – each FADH 2 pumps 2 pairs of H+s, which produces 2 ATP
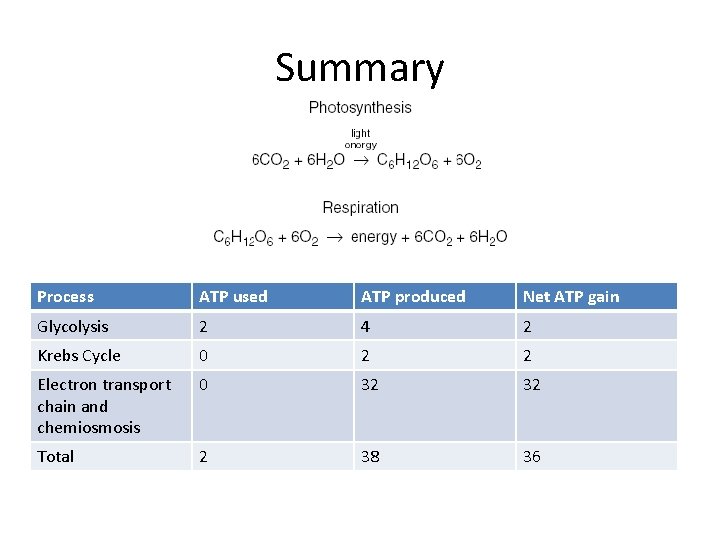
Summary Process ATP used ATP produced Net ATP gain Glycolysis 2 4 2 Krebs Cycle 0 2 2 Electron transport chain and chemiosmosis 0 32 32 Total 2 38 36
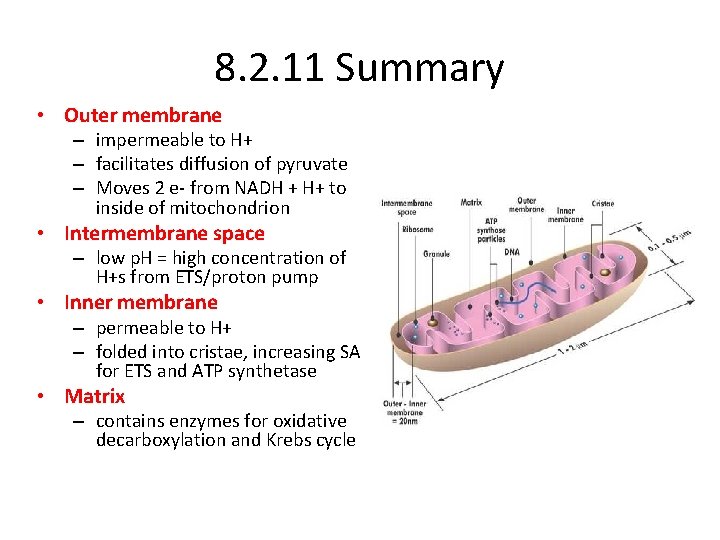
8. 2. 11 Summary • Outer membrane – impermeable to H+ – facilitates diffusion of pyruvate – Moves 2 e- from NADH + H+ to inside of mitochondrion • Intermembrane space – low p. H = high concentration of H+s from ETS/proton pump • Inner membrane – permeable to H+ – folded into cristae, increasing SA for ETS and ATP synthetase • Matrix – contains enzymes for oxidative decarboxylation and Krebs cycle
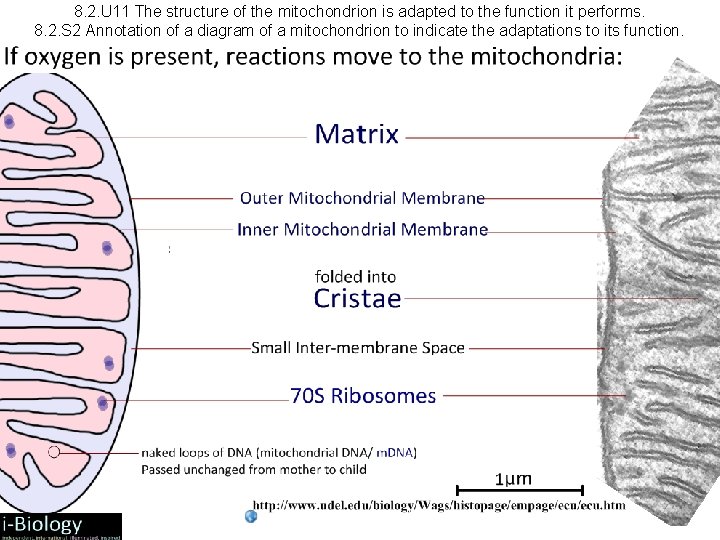
8. 2. U 11 The structure of the mitochondrion is adapted to the function it performs. 8. 2. S 2 Annotation of a diagram of a mitochondrion to indicate the adaptations to its function.
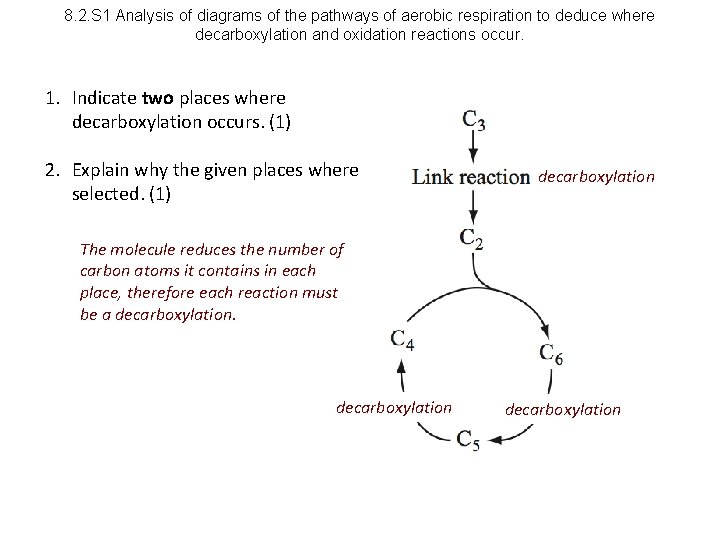
8. 2. S 1 Analysis of diagrams of the pathways of aerobic respiration to deduce where decarboxylation and oxidation reactions occur. 1. Indicate two places where decarboxylation occurs. (1) 2. Explain why the given places where selected. (1) decarboxylation The molecule reduces the number of carbon atoms it contains in each place, therefore each reaction must be a decarboxylation
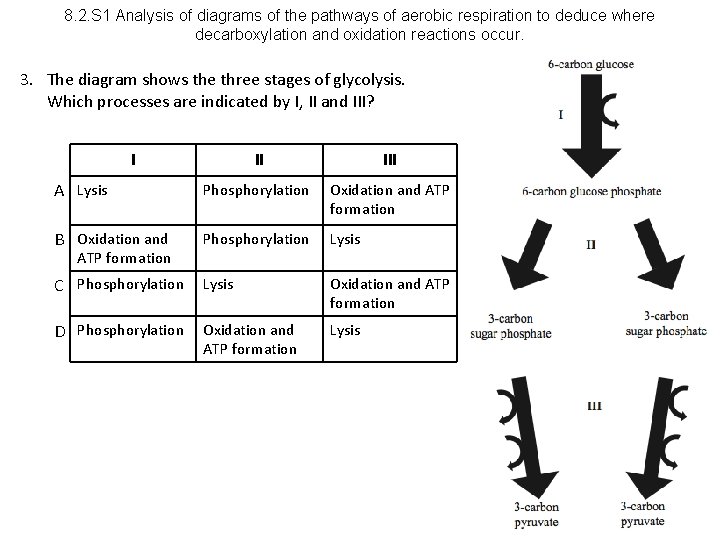
8. 2. S 1 Analysis of diagrams of the pathways of aerobic respiration to deduce where decarboxylation and oxidation reactions occur. 3. The diagram shows the three stages of glycolysis. Which processes are indicated by I, II and III? I II III A Lysis Phosphorylation Oxidation and ATP formation B Oxidation and Phosphorylation Lysis C Phosphorylation Lysis Oxidation and ATP formation D Phosphorylation Oxidation and ATP formation Lysis ATP formation
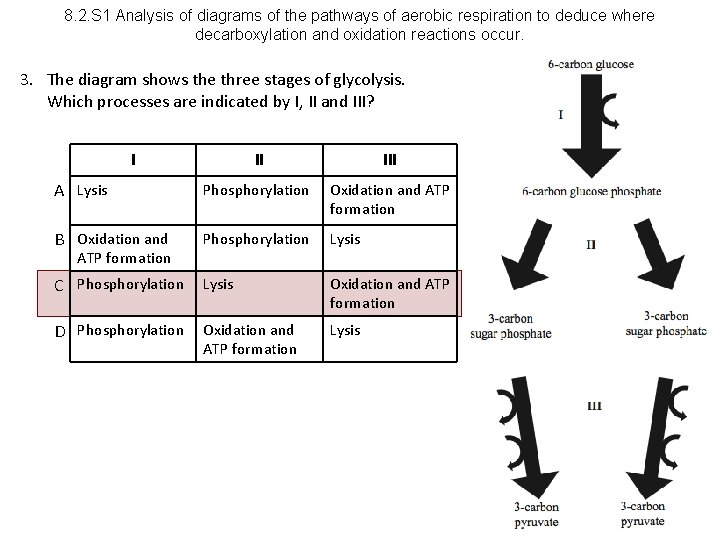
8. 2. S 1 Analysis of diagrams of the pathways of aerobic respiration to deduce where decarboxylation and oxidation reactions occur. 3. The diagram shows the three stages of glycolysis. Which processes are indicated by I, II and III? I II III A Lysis Phosphorylation Oxidation and ATP formation B Oxidation and Phosphorylation Lysis C Phosphorylation Lysis Oxidation and ATP formation D Phosphorylation Oxidation and ATP formation Lysis ATP formation
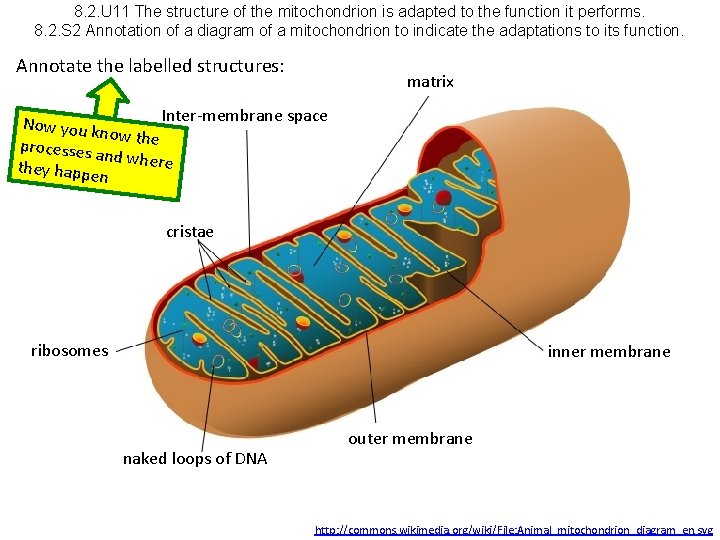
8. 2. U 11 The structure of the mitochondrion is adapted to the function it performs. 8. 2. S 2 Annotation of a diagram of a mitochondrion to indicate the adaptations to its function. Annotate the labelled structures: matrix Inter-membrane space Now you k now the processes a nd where they happe n cristae ribosomes inner membrane naked loops of DNA outer membrane http: //commons. wikimedia. org/wiki/File: Animal_mitochondrion_diagram_en. svg
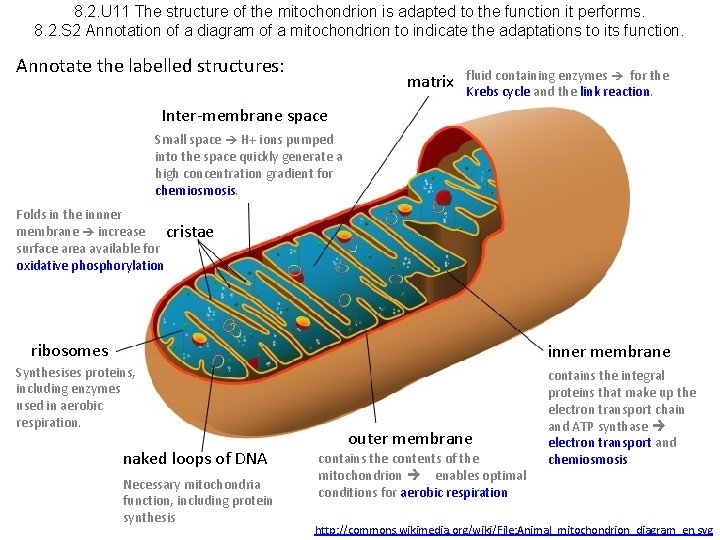
8. 2. U 11 The structure of the mitochondrion is adapted to the function it performs. 8. 2. S 2 Annotation of a diagram of a mitochondrion to indicate the adaptations to its function. Annotate the labelled structures: matrix fluid containing enzymes for the Krebs cycle and the link reaction. Inter-membrane space Small space H+ ions pumped into the space quickly generate a high concentration gradient for chemiosmosis. Folds in the innner membrane increase cristae surface area available for oxidative phosphorylation ribosomes inner membrane Synthesises proteins, including enzymes used in aerobic respiration. naked loops of DNA Necessary mitochondria function, including protein synthesis outer membrane contains the contents of the mitochondrion enables optimal conditions for aerobic respiration contains the integral proteins that make up the electron transport chain and ATP synthase electron transport and chemiosmosis http: //commons. wikimedia. org/wiki/File: Animal_mitochondrion_diagram_en. svg
- Slides: 47