CZECH TECHNICAL UNIVERSITY IN PRAGUE FACULTY OF BIOMEDICAL

CZECH TECHNICAL UNIVERSITY IN PRAGUE FACULTY OF BIOMEDICAL ENGINEERING Förster Energy Transfer Excimer Fluorescence Fluorescent Proteins Martin Hof, Radek Macháň
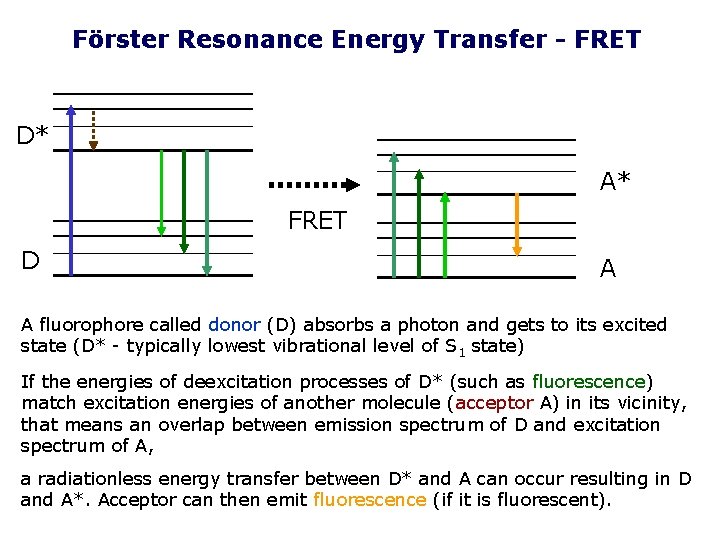
Förster Resonance Energy Transfer - FRET D* A* FRET D A A fluorophore called donor (D) absorbs a photon and gets to its excited state (D* - typically lowest vibrational level of S 1 state) If the energies of deexcitation processes of D* (such as fluorescence) match excitation energies of another molecule (acceptor A) in its vicinity, that means an overlap between emission spectrum of D and excitation spectrum of A, a radiationless energy transfer between D* and A can occur resulting in D and A*. Acceptor can then emit fluorescence (if it is fluorescent).
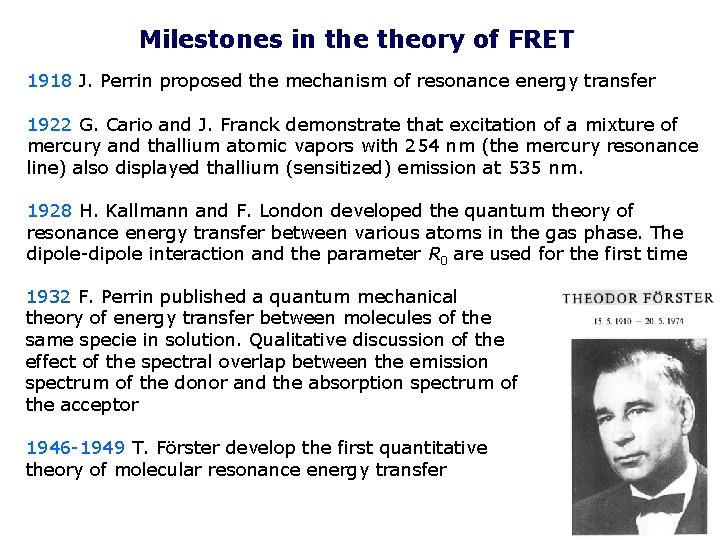
Milestones in theory of FRET 1918 J. Perrin proposed the mechanism of resonance energy transfer 1922 G. Cario and J. Franck demonstrate that excitation of a mixture of mercury and thallium atomic vapors with 254 nm (the mercury resonance line) also displayed thallium (sensitized) emission at 535 nm. 1928 H. Kallmann and F. London developed the quantum theory of resonance energy transfer between various atoms in the gas phase. The dipole-dipole interaction and the parameter R 0 are used for the first time 1932 F. Perrin published a quantum mechanical theory of energy transfer between molecules of the same specie in solution. Qualitative discussion of the effect of the spectral overlap between the emission spectrum of the donor and the absorption spectrum of the acceptor 1946 -1949 T. Förster develop the first quantitative theory of molecular resonance energy transfer
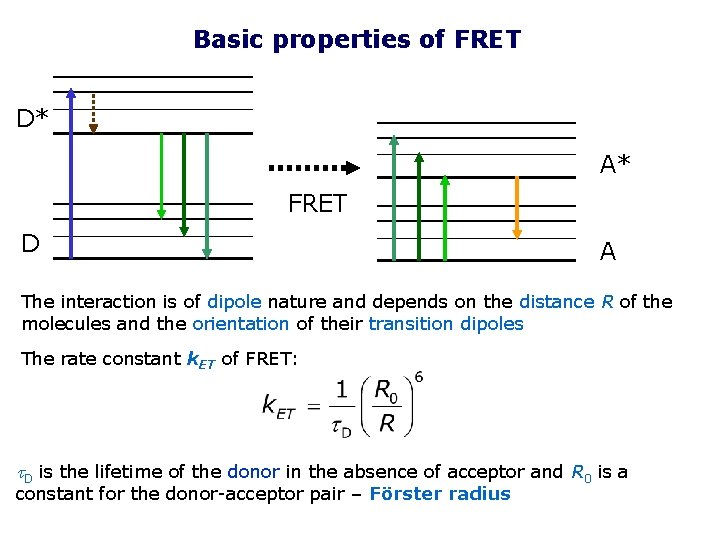
Basic properties of FRET D* A* FRET D A The interaction is of dipole nature and depends on the distance R of the molecules and the orientation of their transition dipoles The rate constant k. ET of FRET: t. D is the lifetime of the donor in the absence of acceptor and R 0 is a constant for the donor-acceptor pair – Förster radius
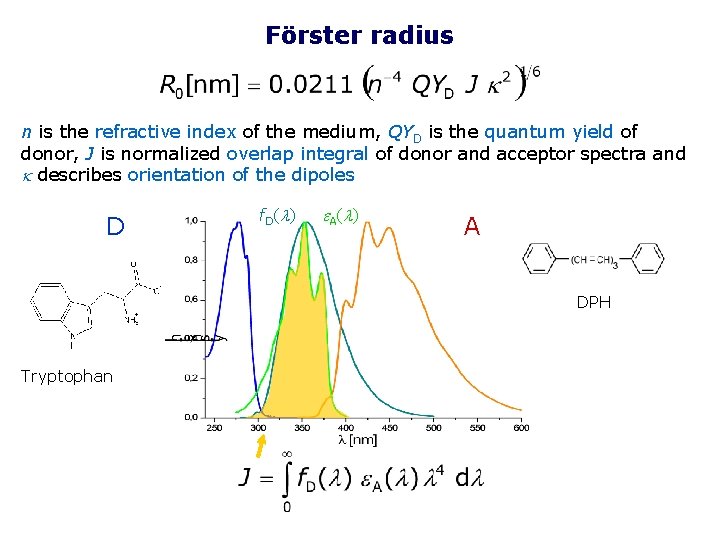
Förster radius n is the refractive index of the medium, QYD is the quantum yield of donor, J is normalized overlap integral of donor and acceptor spectra and k describes orientation of the dipoles D f. D(l) e A (l ) A DPH Tryptophan
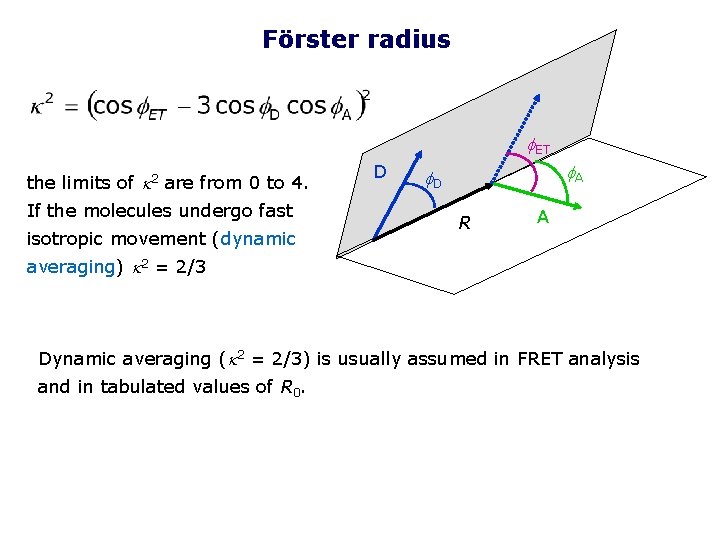
Förster radius f. ET the limits of k 2 are from 0 to 4. If the molecules undergo fast isotropic movement (dynamic D f. A f. D R A averaging) k 2 = 2/3 Dynamic averaging (k 2 = 2/3) is usually assumed in FRET analysis and in tabulated values of R 0.
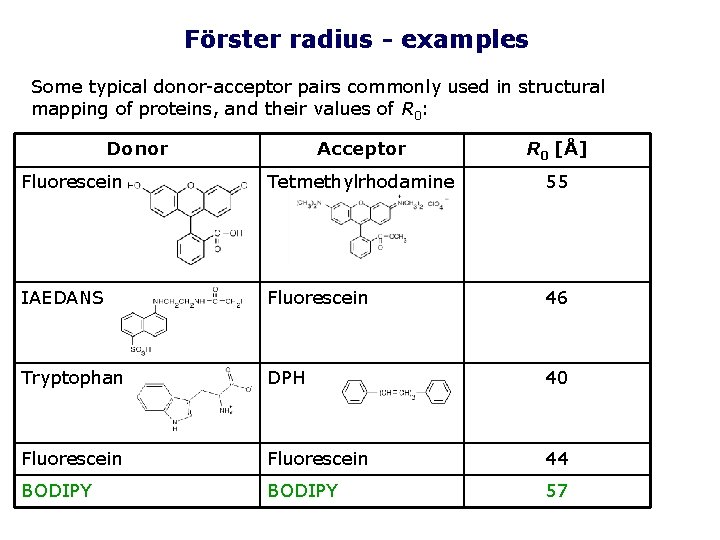
Förster radius - examples Some typical donor-acceptor pairs commonly used in structural mapping of proteins, and their values of R 0: Donor Acceptor R 0 [Å] Fluorescein Tetmethylrhodamine 55 IAEDANS Fluorescein 46 Tryptophan DPH 40 Fluorescein 44 BODIPY 57
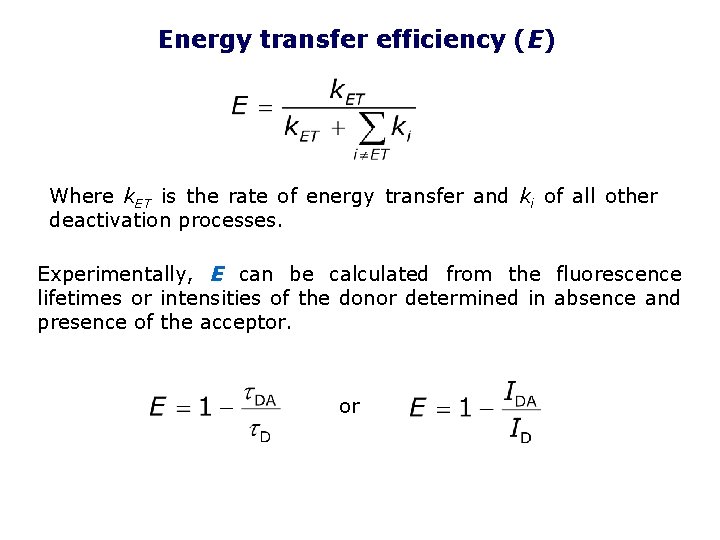
Energy transfer efficiency (E) Where k. ET is the rate of energy transfer and ki of all other deactivation processes. Experimentally, E can be calculated from the fluorescence lifetimes or intensities of the donor determined in absence and presence of the acceptor. or
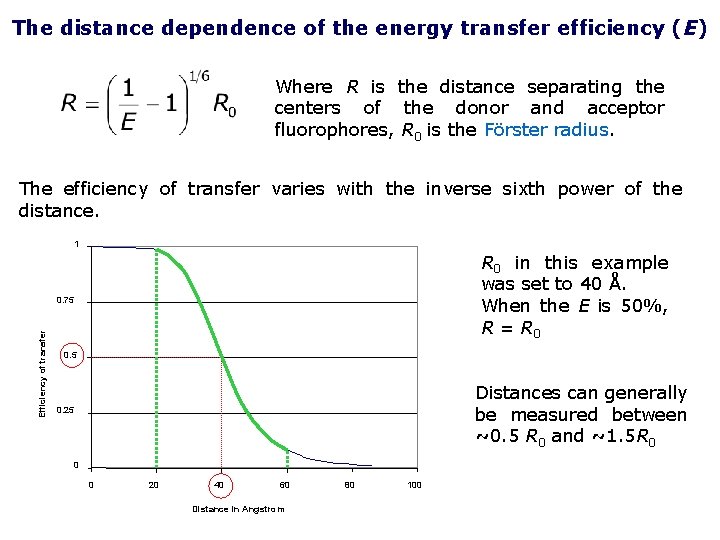
The distance dependence of the energy transfer efficiency (E) Where R is the distance separating the centers of the donor and acceptor fluorophores, R 0 is the Förster radius. The efficiency of transfer varies with the inverse sixth power of the distance. 1 R 0 in this example was set to 40 Å. When the E is 50%, R = R 0 Efficiency of transfer 0. 75 0. 5 Distances can generally be measured between ~0. 5 R 0 and ~1. 5 R 0 0. 25 0 0 20 40 60 Distance in Angstrom 80 100
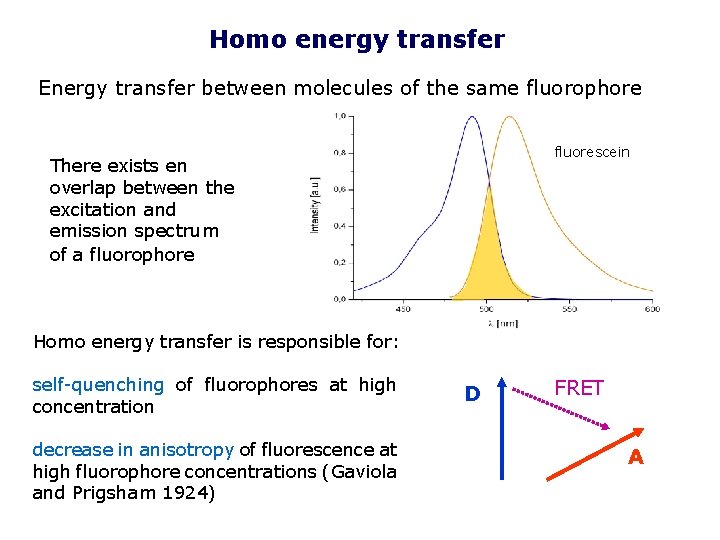
Homo energy transfer Energy transfer between molecules of the same fluorophore fluorescein There exists en overlap between the excitation and emission spectrum of a fluorophore Homo energy transfer is responsible for: self-quenching of fluorophores at high concentration decrease in anisotropy of fluorescence at high fluorophore concentrations (Gaviola and Prigsham 1924) D FRET A
Determination of FRET efficiency Intensity based: • Sensitized emission of the acceptor (provided it is fluorescent) • Decrease in intensity (quenching) of donor fluorescence The main problem of intensity based approaches is the sensitivity to donor and acceptor concentration Kinetic based: • Decrease in lifetime (quenching) of donor fluorescence • Fluorescence decay of acceptor - It contains a rise in the initial phase corresponding to the kinetics of donor deexcitation by FRET (a component with “negative amplitude” in the fitted decay) • Kinetics of donor photobleaching The use of donor fluorescence is usually preferred, because the acceptor is usually to some extent excitable by the excitation wavelength of the donor – only a part of acceptor fluorescence is a result of FRET
Photobleaching of donor Photobleaching is a decrease in fluorescence intensity due to permanent inactivation of the fluorophores. It is usually caused by excited state reactions of the fluorophore in triplet state (for example with oxygen). Photobleaching is observed mainly at high excitation intensities when a significant fraction of molecules undergoes intersystem crossing. FRET represents an additional deexcitation channel and, thus decreases the probability of intersystem crossing and photobleaching. The decrease of intensity due to photobleaching is, therefore, slower where t. PB is the intensity decay time due to photobleaching (I ~ exp(-t/t. PB)) Photobleaching measurement is not sensitive to concentration and it does not require high temporal resolution – steady-state instrumentation)
FRET and distance measurements ü FRET can be used to obtain structural maps of complex biological structures, primarily proteins or other macromolecular assemblies. ü Measurements of energy transfer can provide intra- or intermolecular distances for proteins and their ligands in the range of 10 -100 Å. ü FRET can detect change in distance (1 -2 Å) between loci in proteins, hence it is a sensitive measure of conformational changes. m The donor and acceptor must be within 0. 5 R 0 - 1. 5 R 0 from each other. m These measurements give the average distance between the two fluorophores. When measuring a change in distance, the result gives no indication of which fluorophore moves. m Experiments can be done with different donor-acceptor pairs. If the same distance is obtained, the result is very likely correct. m Quantitative analysis is restricted to the cases where only a few donors and acceptors are present
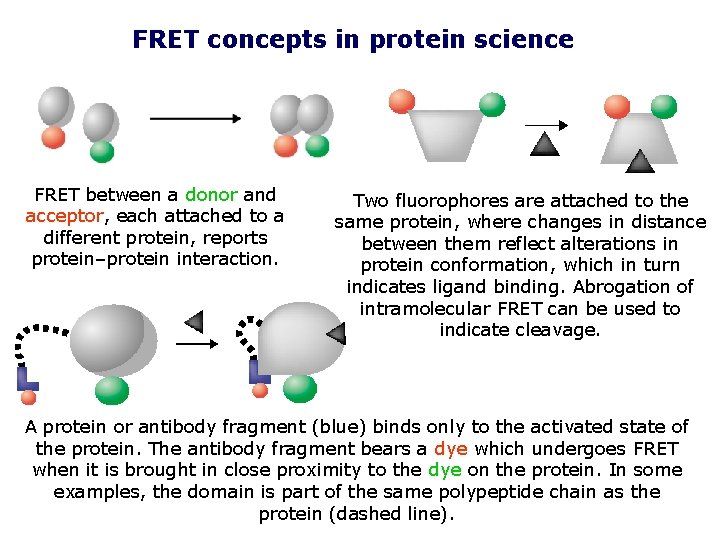
FRET concepts in protein science FRET between a donor and acceptor, each attached to a different protein, reports protein–protein interaction. Two fluorophores are attached to the same protein, where changes in distance between them reflect alterations in protein conformation, which in turn indicates ligand binding. Abrogation of intramolecular FRET can be used to indicate cleavage. A protein or antibody fragment (blue) binds only to the activated state of the protein. The antibody fragment bears a dye which undergoes FRET when it is brought in close proximity to the dye on the protein. In some examples, the domain is part of the same polypeptide chain as the protein (dashed line).
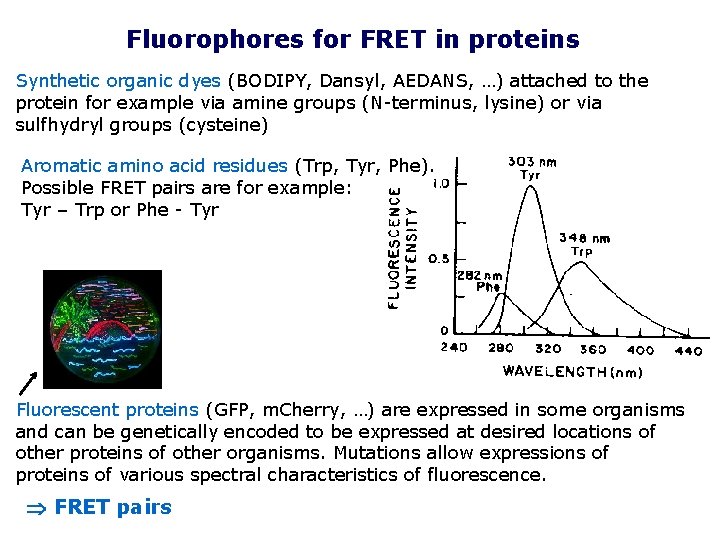
Fluorophores for FRET in proteins Synthetic organic dyes (BODIPY, Dansyl, AEDANS, …) attached to the protein for example via amine groups (N-terminus, lysine) or via sulfhydryl groups (cysteine) Aromatic amino acid residues (Trp, Tyr, Phe). Possible FRET pairs are for example: Tyr – Trp or Phe - Tyr Fluorescent proteins (GFP, m. Cherry, …) are expressed in some organisms and can be genetically encoded to be expressed at desired locations of other proteins of other organisms. Mutations allow expressions of proteins of various spectral characteristics of fluorescence. FRET pairs
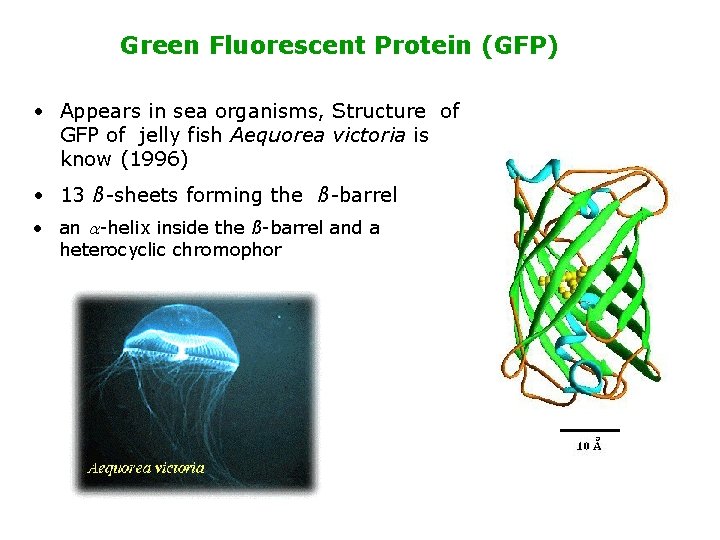
Green Fluorescent Protein (GFP) • Appears in sea organisms, Structure of GFP of jelly fish Aequorea victoria is know (1996) • 13 ß-sheets forming the ß-barrel • an a-helix inside the ß-barrel and a heterocyclic chromophor
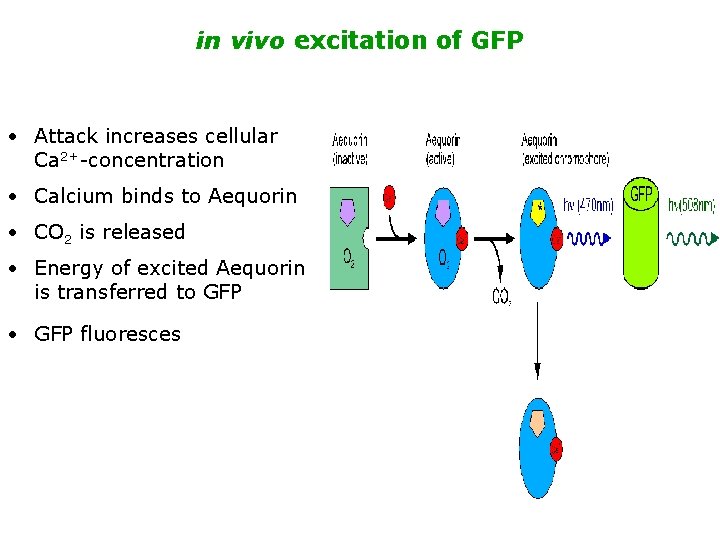
in vivo excitation of GFP • Attack increases cellular Ca 2+-concentration • Calcium binds to Aequorin • CO 2 is released • Energy of excited Aequorin is transferred to GFP • GFP fluoresces
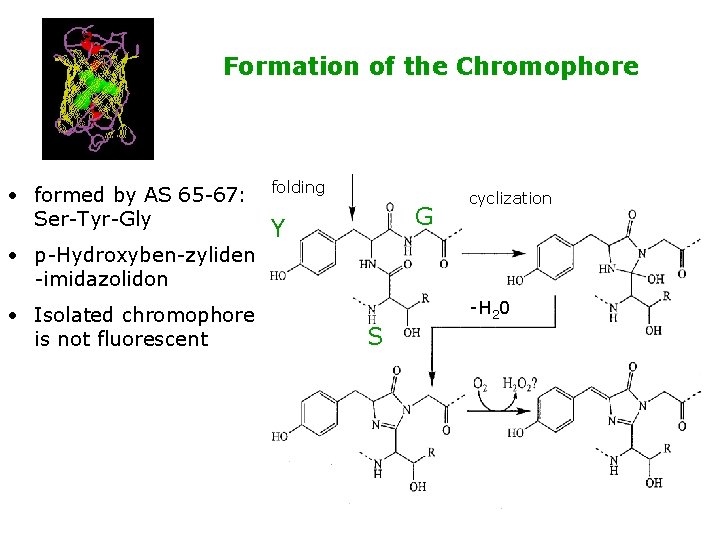
Formation of the Chromophore • formed by AS 65 -67: Ser-Tyr-Gly • p-Hydroxyben-zyliden -imidazolidon • Isolated chromophore is not fluorescent folding G Y cyclization -H 20 S
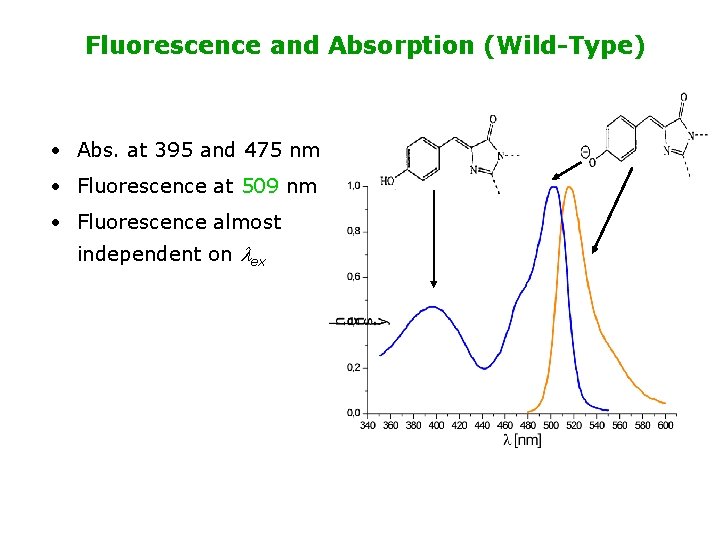
Fluorescence and Absorption (Wild-Type) • Abs. at 395 and 475 nm • Fluorescence at 509 nm • Fluorescence almost independent on lex
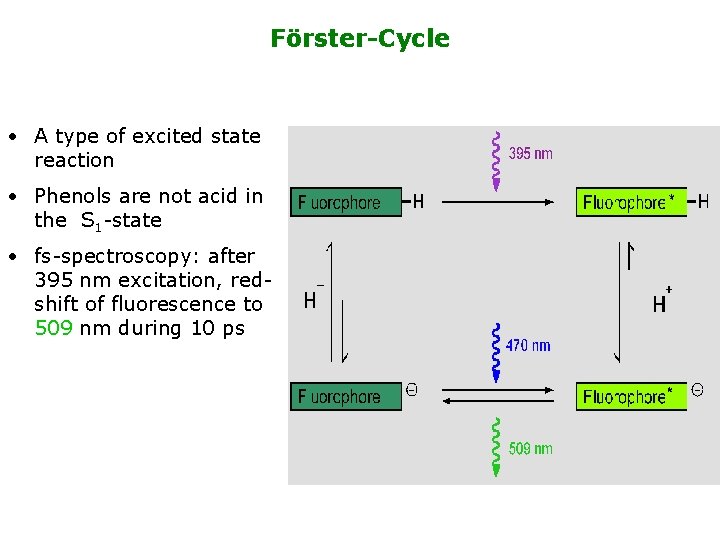
Förster-Cycle • A type of excited state reaction • Phenols are not acid in the S 1 -state • fs-spectroscopy: after 395 nm excitation, redshift of fluorescence to 509 nm during 10 ps
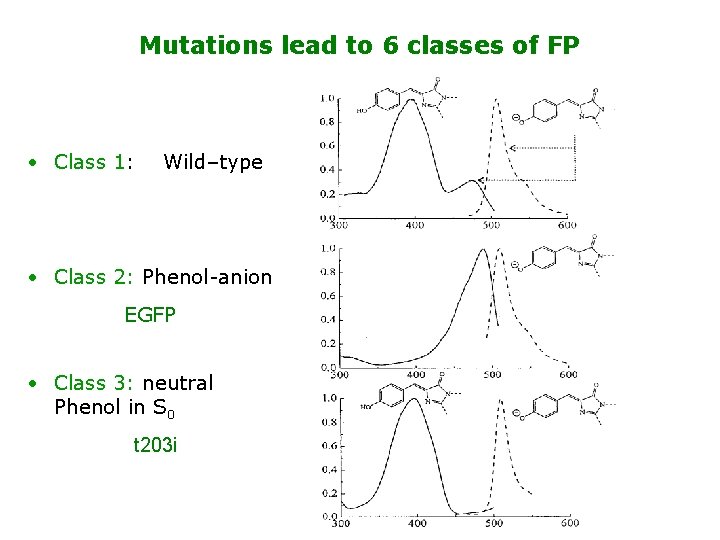
Mutations lead to 6 classes of FP • Class 1: Wild–type • Class 2: Phenol-anion EGFP • Class 3: neutral Phenol in S 0 t 203 i
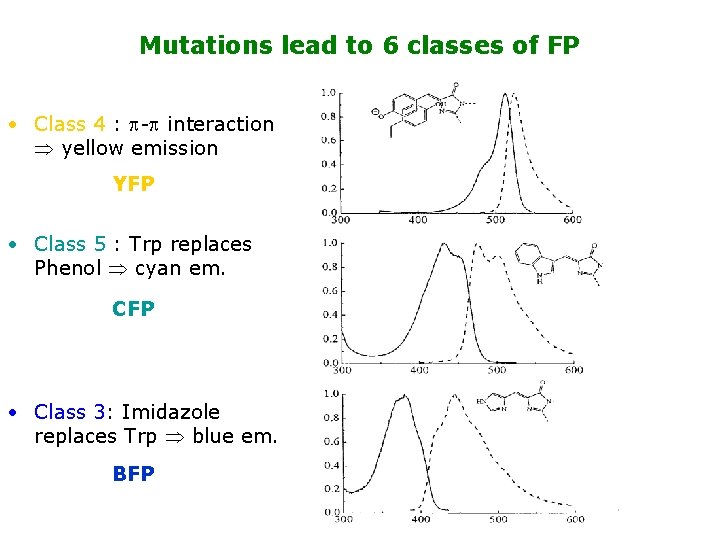
Mutations lead to 6 classes of FP • Class 4 : - interaction yellow emission YFP • Class 5 : Trp replaces Phenol cyan em. CFP • Class 3: Imidazole replaces Trp blue em. BFP
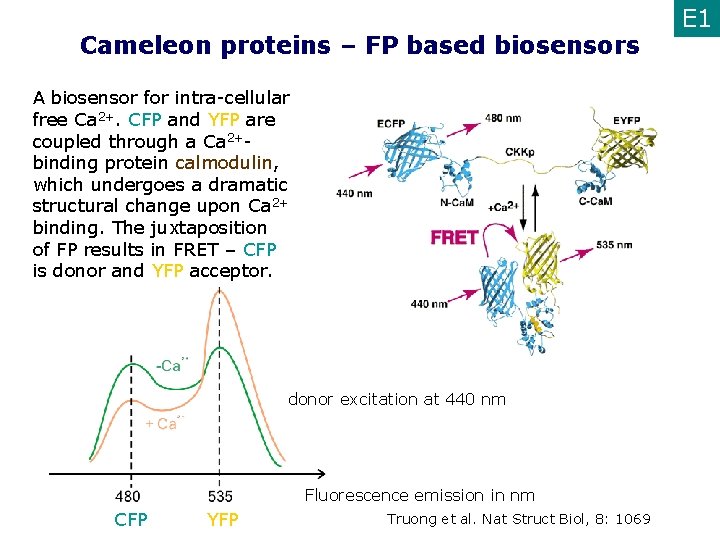
Cameleon proteins – FP based biosensors A biosensor for intra-cellular free Ca 2+. CFP and YFP are coupled through a Ca 2+binding protein calmodulin, which undergoes a dramatic structural change upon Ca 2+ binding. The juxtaposition of FP results in FRET – CFP is donor and YFP acceptor. donor excitation at 440 nm Fluorescence emission in nm CFP YFP Truong et al. Nat Struct Biol, 8: 1069 E 1
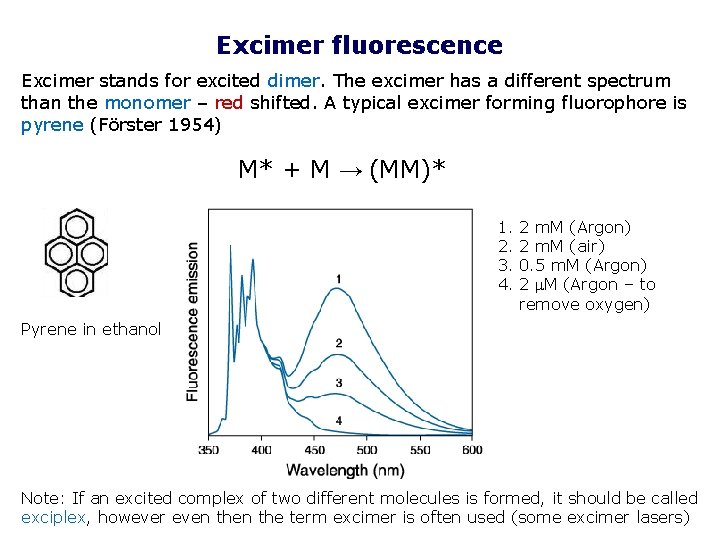
Excimer fluorescence Excimer stands for excited dimer. The excimer has a different spectrum than the monomer – red shifted. A typical excimer forming fluorophore is pyrene (Förster 1954) M* + M → (MM)* 1. 2. 3. 4. 2 m. M (Argon) 2 m. M (air) 0. 5 m. M (Argon) 2 M (Argon – to remove oxygen) Pyrene in ethanol Note: If an excited complex of two different molecules is formed, it should be called exciplex, however even the term excimer is often used (some excimer lasers)
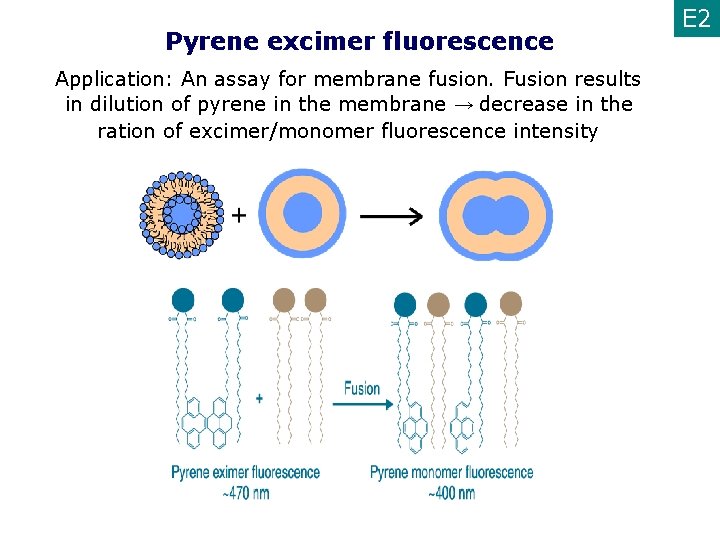
Pyrene excimer fluorescence Application: An assay for membrane fusion. Fusion results in dilution of pyrene in the membrane → decrease in the ration of excimer/monomer fluorescence intensity E 2
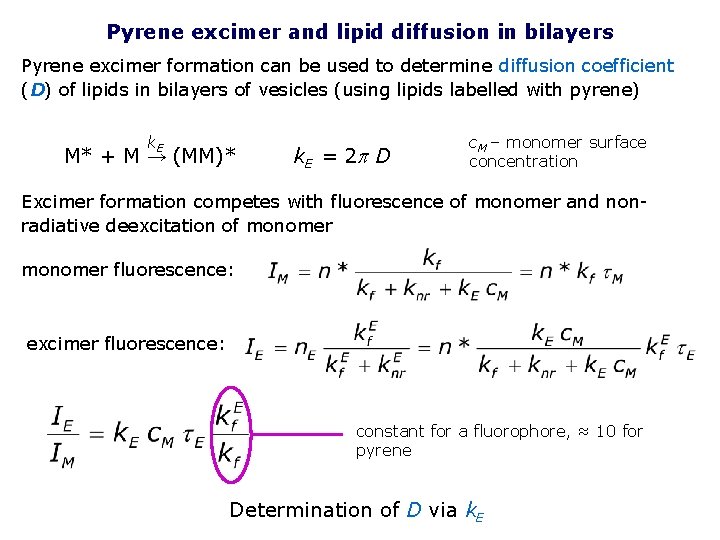
Pyrene excimer and lipid diffusion in bilayers Pyrene excimer formation can be used to determine diffusion coefficient (D) of lipids in bilayers of vesicles (using lipids labelled with pyrene) k. E M* + M → (MM)* k. E = 2 p D c. M – monomer surface concentration Excimer formation competes with fluorescence of monomer and nonradiative deexcitation of monomer fluorescence: excimer fluorescence: constant for a fluorophore, ≈ 10 for pyrene Determination of D via k. E

Acknowledgement The course was inspired by courses of: Prof. David M. Jameson, Ph. D. Prof. RNDr. Jaromír Plášek, Csc. Prof. William Reusch Financial support from the grant: FRVŠ 33/119970
- Slides: 27