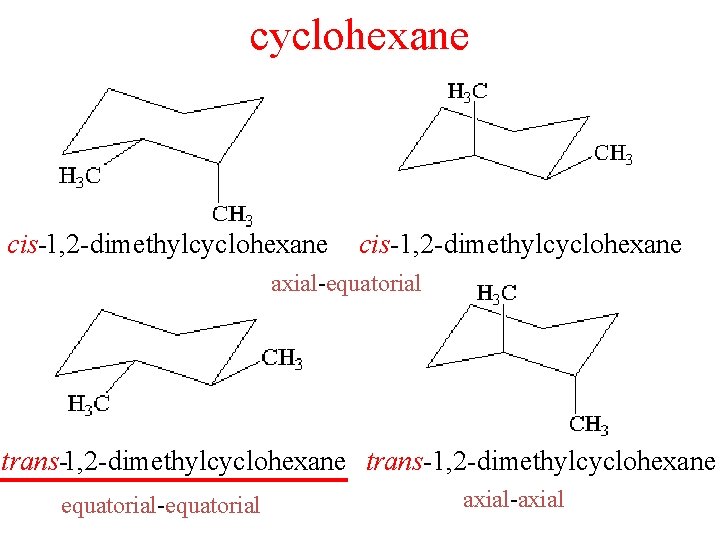
cyclohexane cis1 2 dimethylcyclohexane axialequatorial trans1 2 dimethylcyclohexane

- Slides: 20

cyclohexane cis-1, 2 -dimethylcyclohexane axial-equatorial trans-1, 2 -dimethylcyclohexane equatorial-equatorial axial-axial

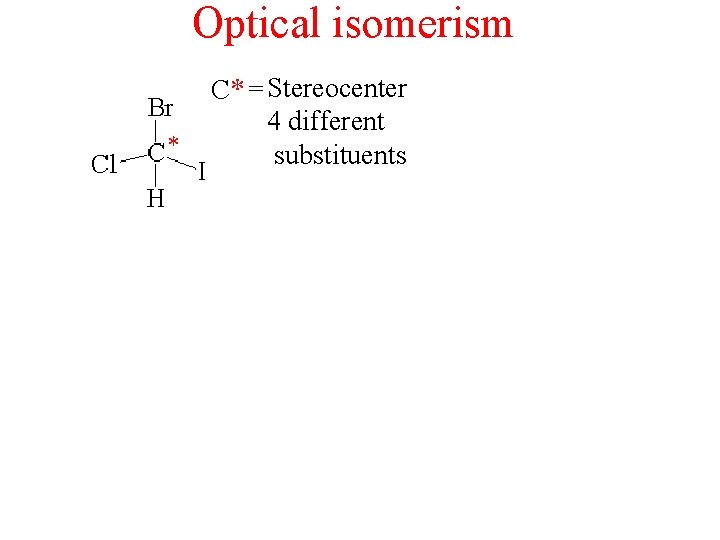
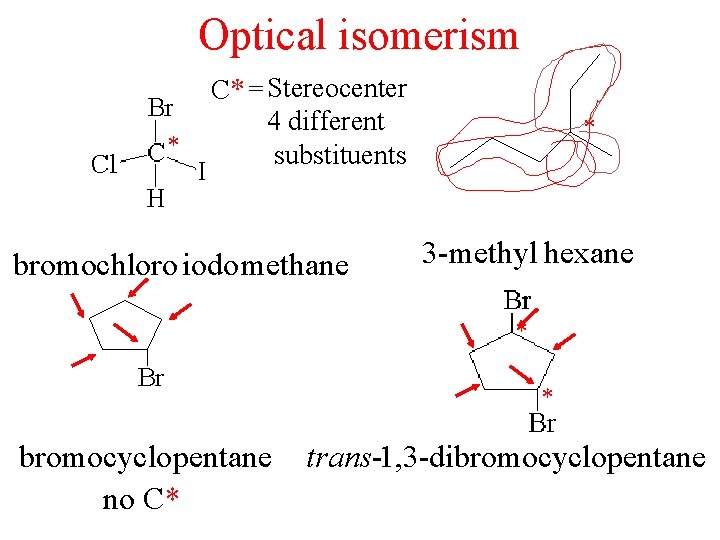
Optical isomerism * C* = Stereocenter 4 different substituents

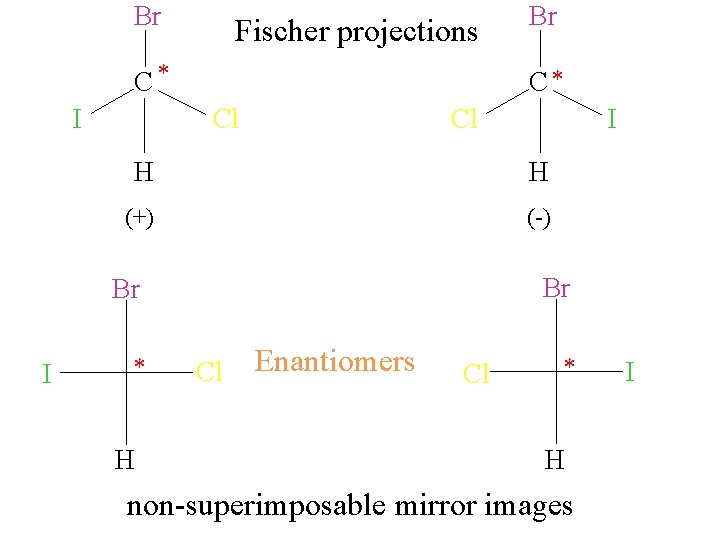
Br Fischer projections C* I C* Cl Cl I H H (+) (-) Br Br I Br * H Cl Enantiomers Cl * H non-superimposable mirror images I

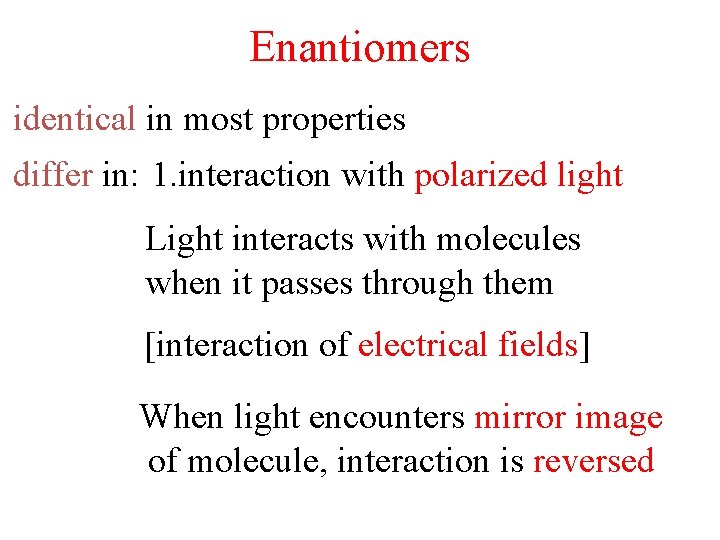
Enantiomers identical in most properties differ in: 1. interaction with polarized light 2. interaction with chiral environments Light interacts with molecules when it passes through them [interaction of electrical fields] When light encounters mirror image of molecule, interaction is reversed

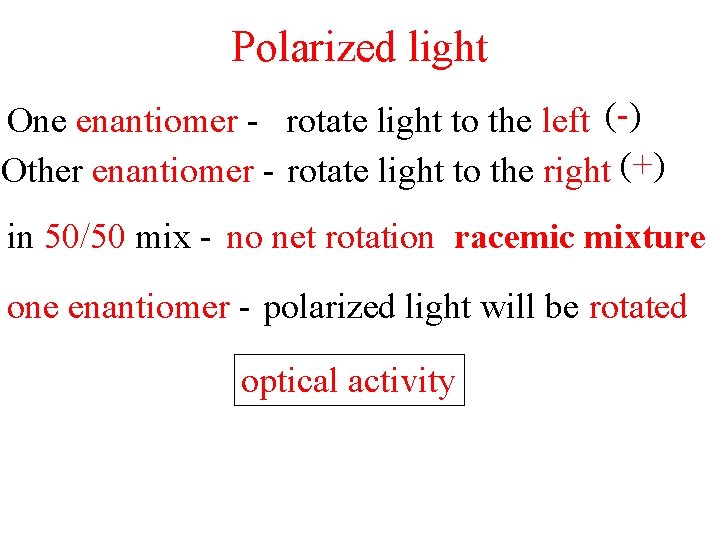
Polarized light One enantiomer - rotate light to the left (-) Other enantiomer - rotate light to the right (+) in 50/50 mix - no net rotation racemic mixture one enantiomer - polarized light will be rotated optical activity

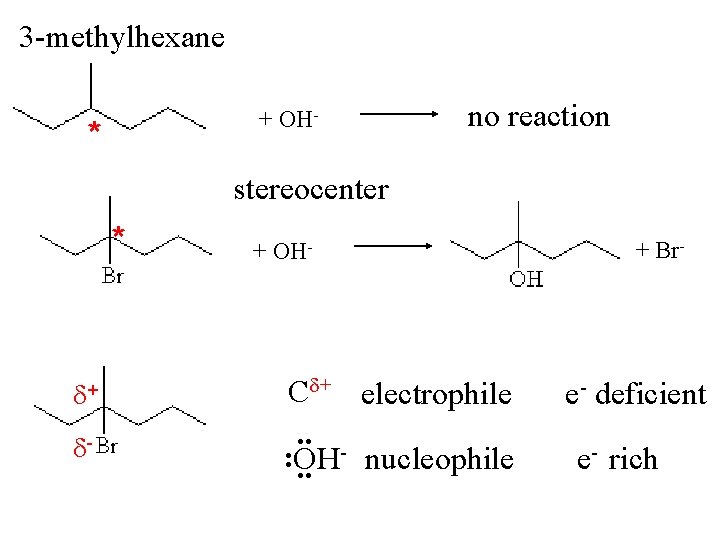
Optical isomerism * C* = Stereocenter 4 different substituents bromochloro iodomethane * 3 -methyl hexane * * bromocyclopentane no C* trans-1, 3 -dibromocyclopentane

Alkane Summary 1. Alkanes - sp 3 hybridized 2. Relatively unreactive Substitution with halogens Combustion 3. Non-polar IMF = London Dispersion Forces size structure

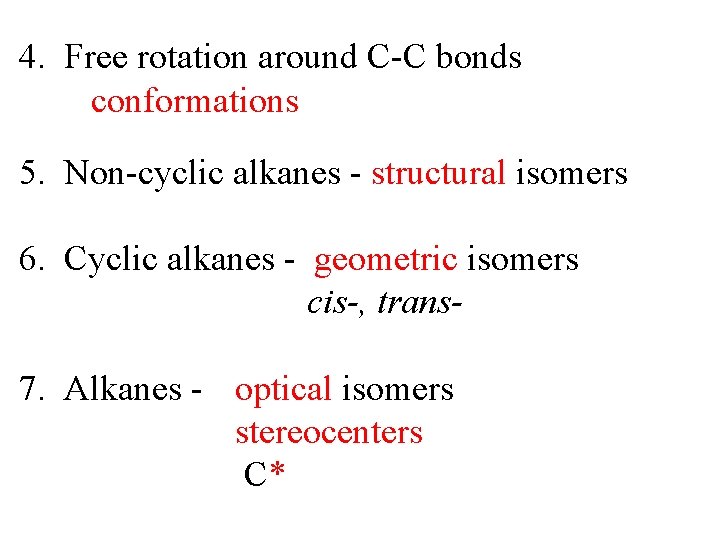
4. Free rotation around C-C bonds conformations 5. Non-cyclic alkanes - structural isomers 6. Cyclic alkanes - geometric isomers cis-, trans 7. Alkanes - optical isomers stereocenters C*

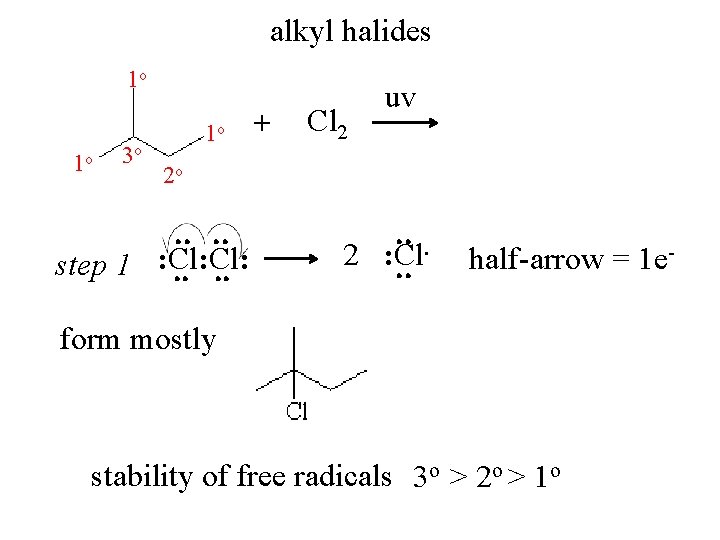
alkyl halides 1 o 1 o 3 o 1 o + Cl 2 uv 2 o . . step 1 : Cl: . . . . 2 : Cl. . half-arrow = 1 e- form mostly stability of free radicals 3 o > 2 o > 1 o

3 -methylhexane + OH- * no reaction stereocenter * + - + OH- C + electrophile. . : OH nucleophile. . + Br- e- deficient e- rich

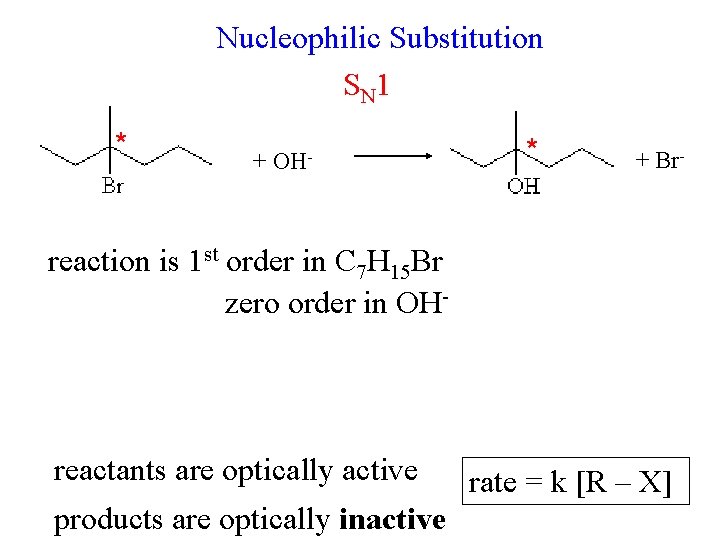
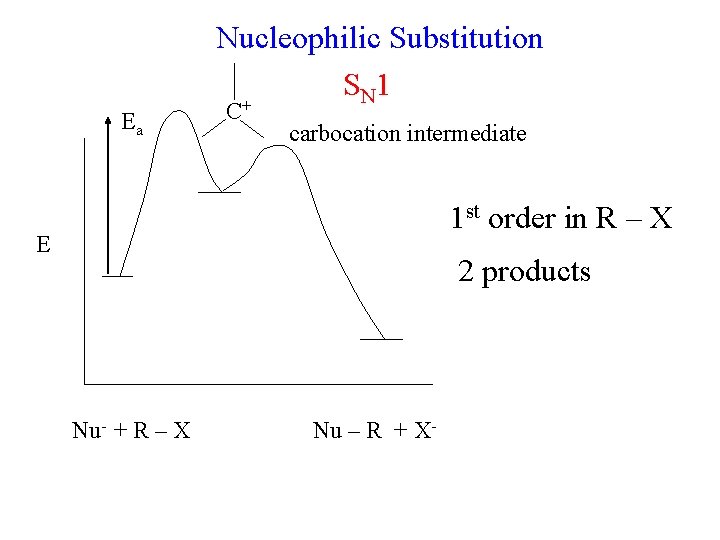
Nucleophilic Substitution S N 1 * + OH- * + Br- reaction is 1 st order in C 7 H 15 Br zero order in OH- reactants are optically active products are optically inactive rate = k [R – X]

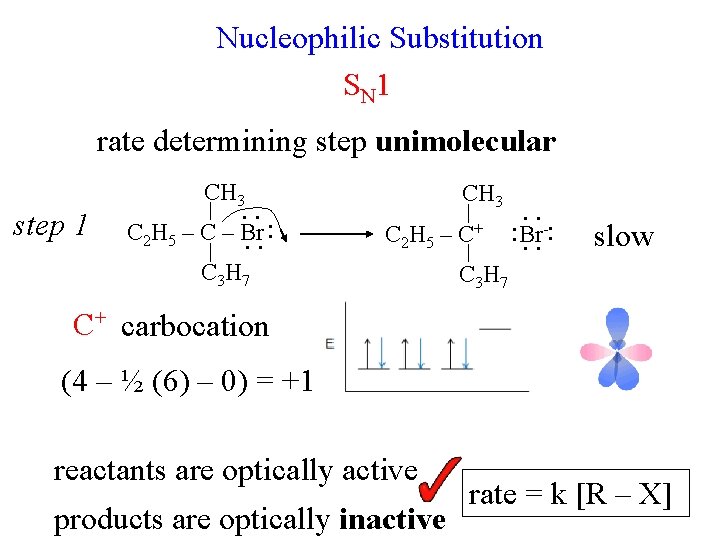
Nucleophilic Substitution S N 1 rate determining step unimolecular step 1 CH 3. . C 2 H 5 – C – Br. . : C 3 H 7 CH 3 C 2 H 5 – C+ . . -: : Br. . slow C 3 H 7 C+ carbocation (4 – ½ (6) – 0) = +1 reactants are optically active products are optically inactive rate = k [R – X]

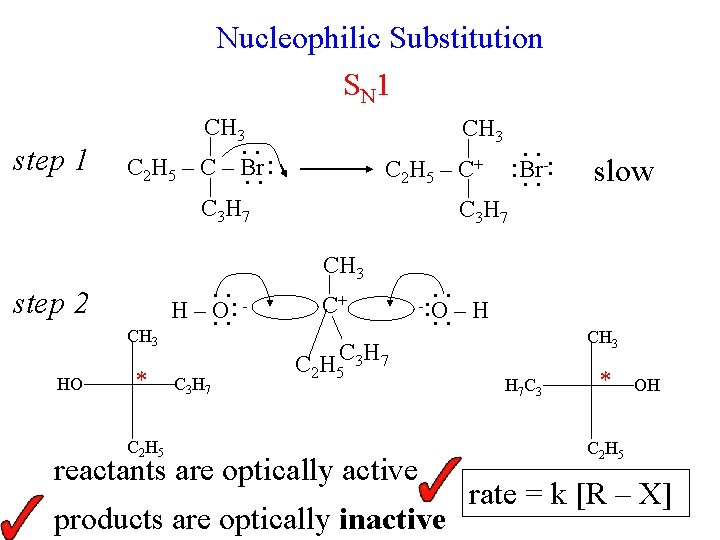
Nucleophilic Substitution S N 1 step 1 CH 3. . C 2 H 5 – C – Br. . : C 3 H 7 step 2 CH 3 HO * . . : H–O. . C 3 H 7 CH 3 . . -: : Br. . C 2 H 5 – C + slow C 3 H 7 CH 3 C+ . . – H - : O C 2 H 5 C 3 H 7 C 2 H 5 reactants are optically active products are optically inactive CH 3 H 7 C 3 * OH C 2 H 5 rate = k [R – X]

Nucleophilic Substitution S N 1 Ea C+ carbocation intermediate 1 st order in R – X E 2 products Nu- + R – X Nu – R + X-

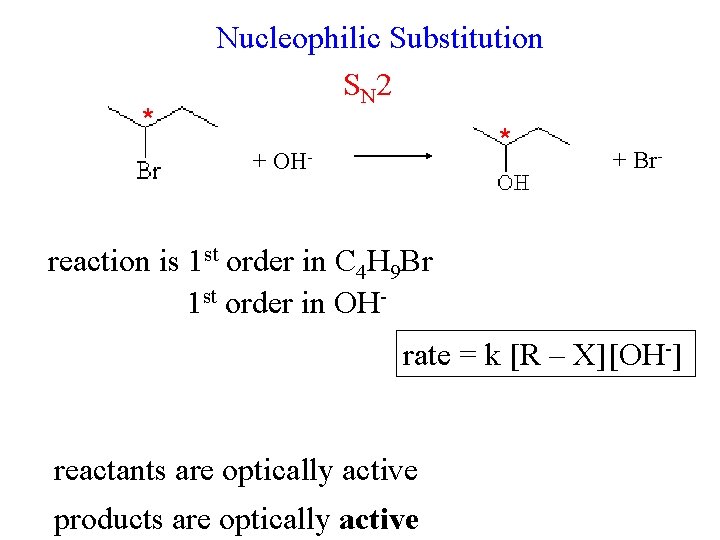
* Nucleophilic Substitution S N 2 + * OH- + Br- reaction is 1 st order in C 4 H 9 Br 1 st order in OHrate = k [R – X] [OH-] reactants are optically active products are optically active

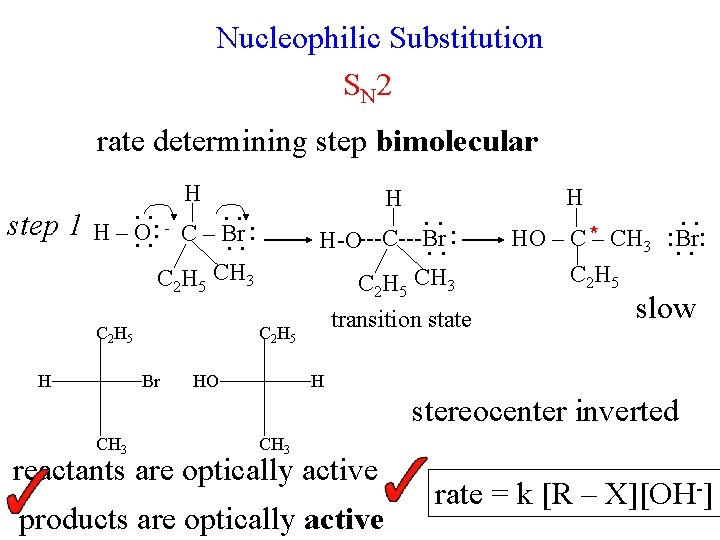
Nucleophilic Substitution S N 2 rate determining step bimolecular H step 1 H . . H–O. . : C – Br. . : C 2 H 5 CH 3 C 2 H 5 H C 2 H 5 Br HO . . H-O---C---Br. . : C 2 H 5 CH 3 transition state H HO – C *– CH 3 C 2 H 5 . . : Br: . . slow H stereocenter inverted CH 3 reactants are optically active products are optically active rate = k [R – X][OH-] -

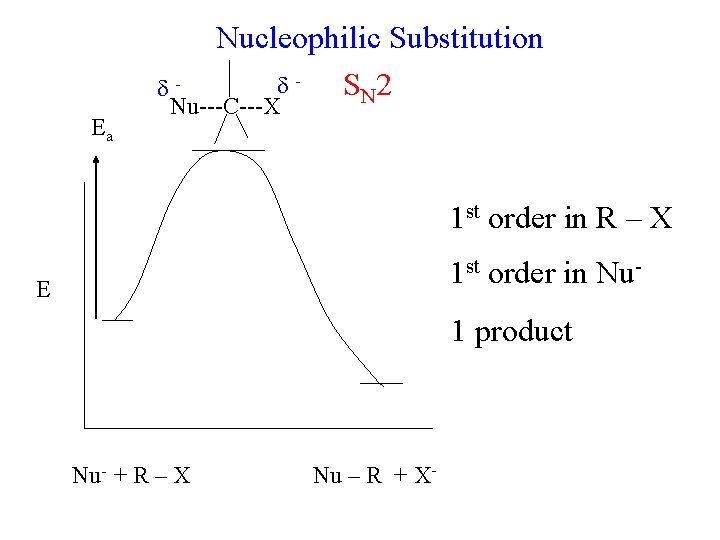
Nucleophilic Substitution S N 2 Ea Nu---C---X 1 st order in R – X 1 st order in Nu- E 1 product Nu- + R – X Nu – R + X-

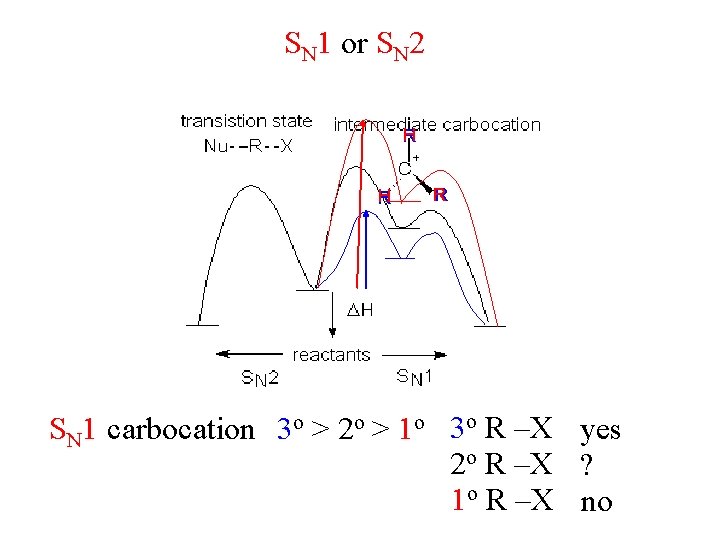
SN 1 or SN 2 H R R SN 1 carbocation 3 o > 2 o > 1 o 3 o R –X yes 2 o R –X ? 1 o R –X no

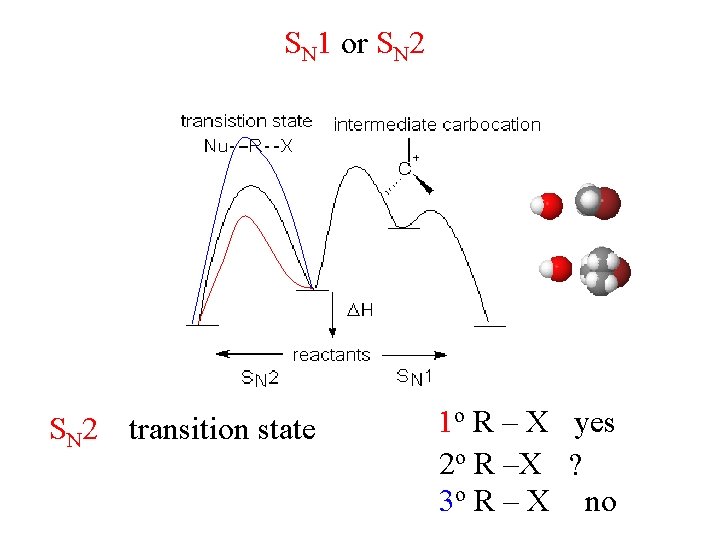
SN 1 or SN 2 transition state 1 o R – X yes 2 o R –X ? 3 o R – X no

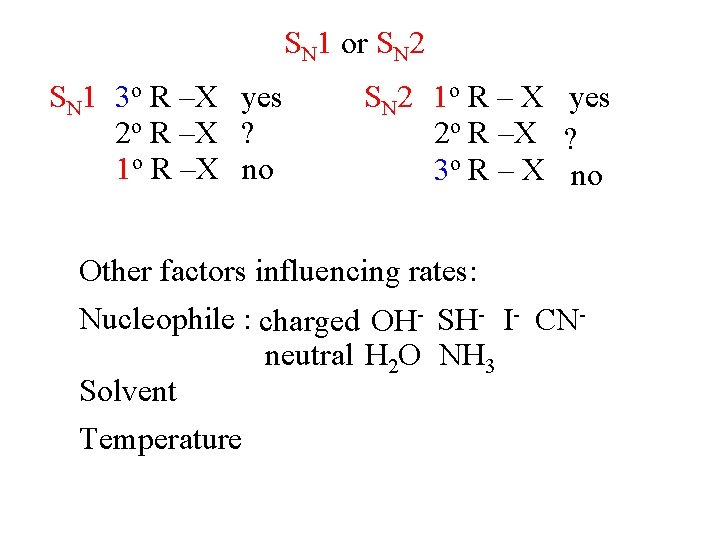
SN 1 or SN 2 SN 1 3 o R –X yes 2 o R –X ? 1 o R –X no SN 2 1 o R – X yes 2 o R –X ? 3 o R – X no Other factors influencing rates: Nucleophile : charged OH- SH- I- CNneutral H 2 O NH 3 Solvent Temperature