Cycling of Matter in Ecosystems Recycling Matter All







- Slides: 18

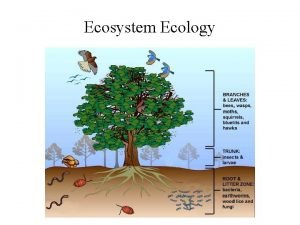
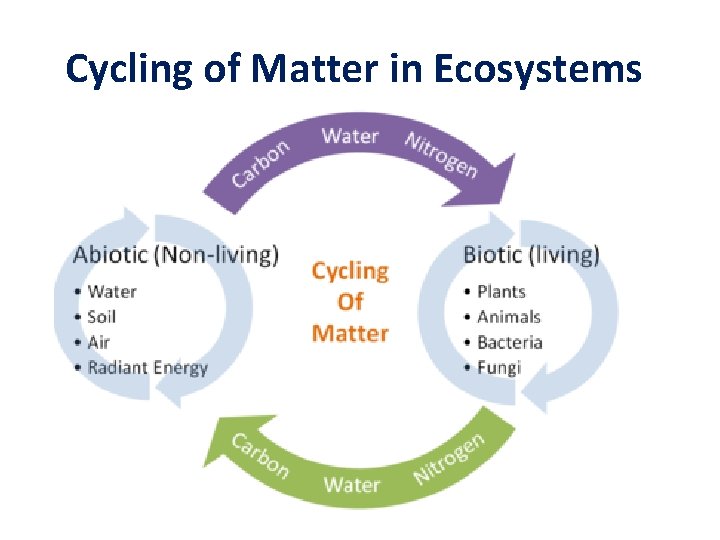
Cycling of Matter in Ecosystems

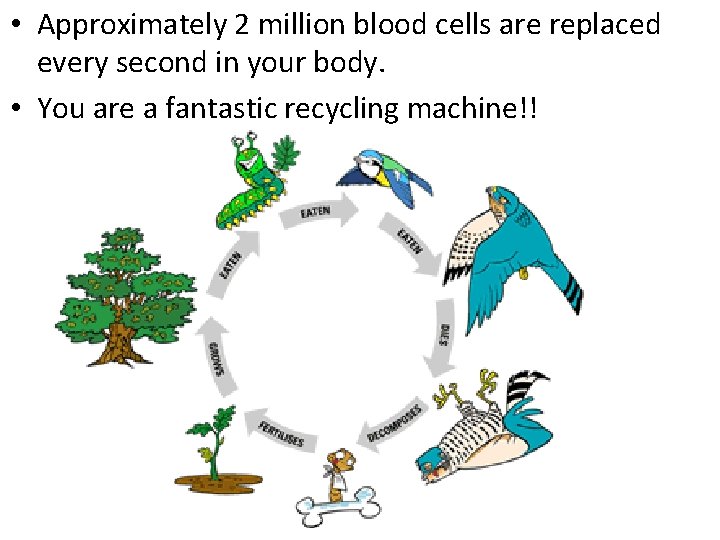
Recycling Matter • All life on Earth requires water and nutrients • These particles of matter don’t remain in your body forever • Every part of every cell in your body is replaced over time • We are like living recycling machines • Nutrients from food are used to repair and renew all the cells in our body

• Approximately 2 million blood cells are replaced every second in your body. • You are a fantastic recycling machine!!

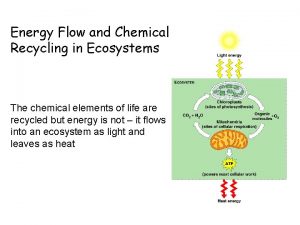
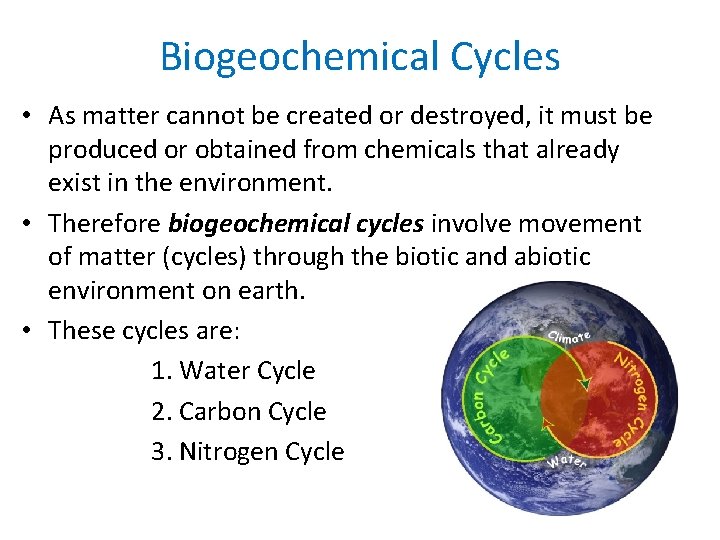
Biogeochemical Cycles • As matter cannot be created or destroyed, it must be produced or obtained from chemicals that already exist in the environment. • Therefore biogeochemical cycles involve movement of matter (cycles) through the biotic and abiotic environment on earth. • These cycles are: 1. Water Cycle 2. Carbon Cycle 3. Nitrogen Cycle

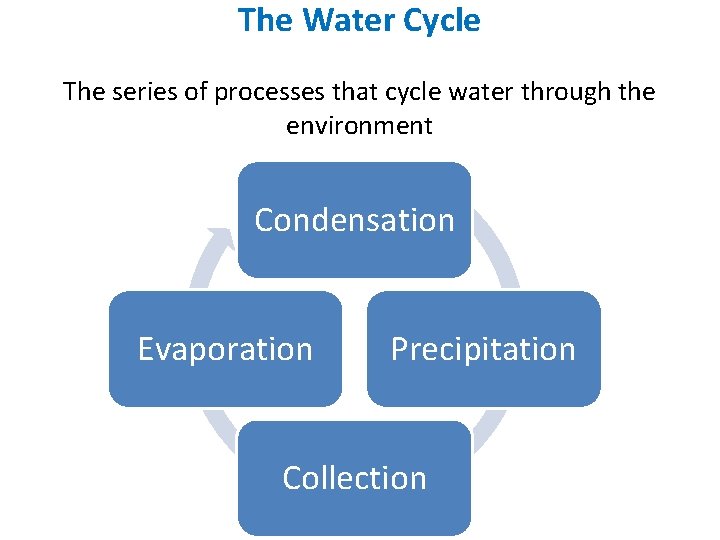
The Water Cycle The series of processes that cycle water through the environment Condensation Evaporation Precipitation Collection


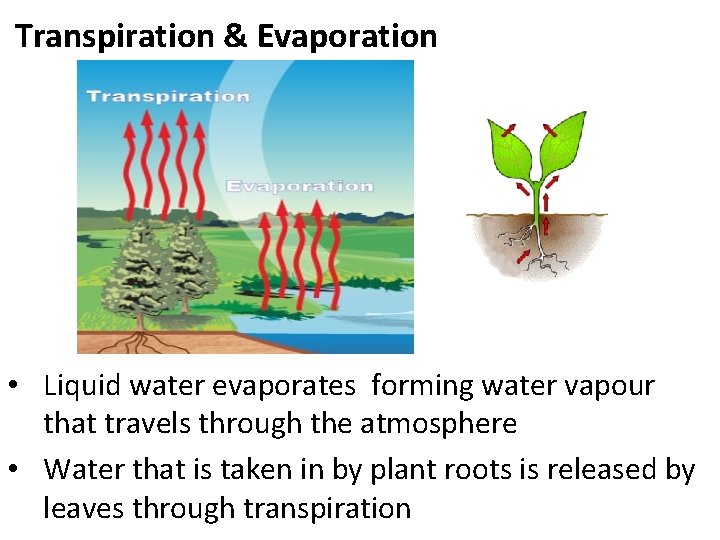
Transpiration & Evaporation • Liquid water evaporates forming water vapour that travels through the atmosphere • Water that is taken in by plant roots is released by leaves through transpiration

Condensation • The vapour eventually condenses • It forms liquid water or ice crystals in the atmosphere

Precipitation and Runoff • Vapour returns to the Earth as rain, hail or snow • Water falling on land melting snow move across the surface (runoff) and enter bodies of water • If this doesn’t occur it will enter the soil and groundwater instead

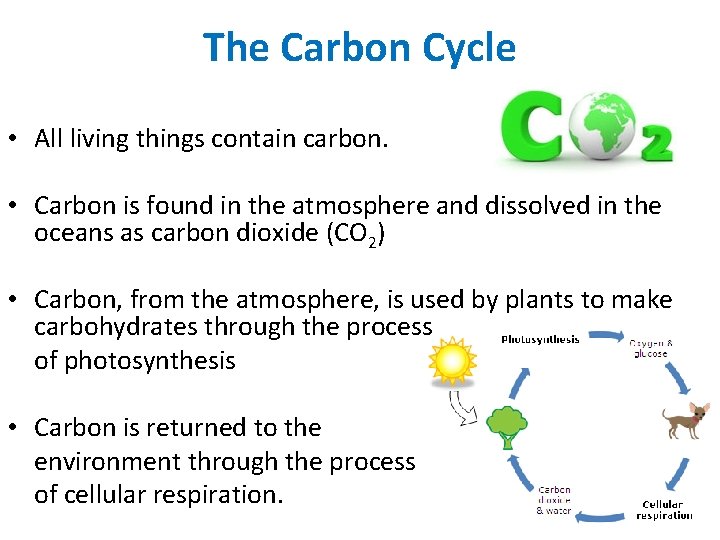
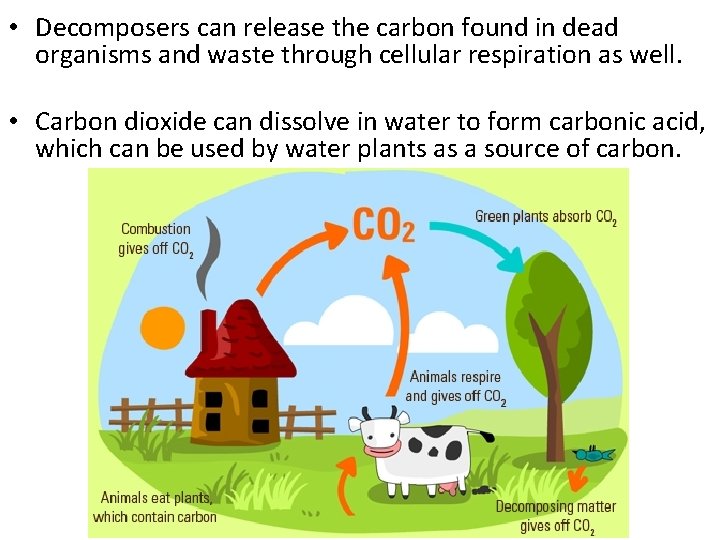
The Carbon Cycle • All living things contain carbon. • Carbon is found in the atmosphere and dissolved in the oceans as carbon dioxide (CO 2) • Carbon, from the atmosphere, is used by plants to make carbohydrates through the process of photosynthesis • Carbon is returned to the environment through the process of cellular respiration.

• Decomposers can release the carbon found in dead organisms and waste through cellular respiration as well. • Carbon dioxide can dissolve in water to form carbonic acid, which can be used by water plants as a source of carbon.


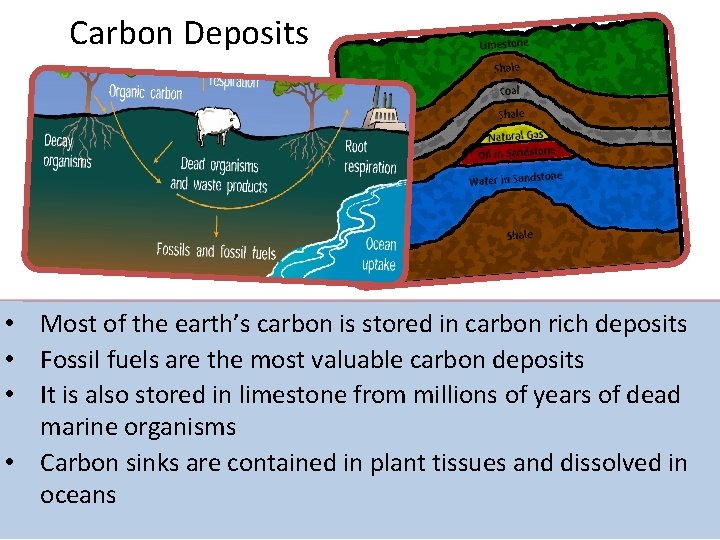
Carbon Deposits • Most of the earth’s carbon is stored in carbon rich deposits • Fossil fuels are the most valuable carbon deposits • It is also stored in limestone from millions of years of dead marine organisms • Carbon sinks are contained in plant tissues and dissolved in oceans

Human Activities and the Carbon Cycle • Humans increase the concentration of carbon dioxide in the atmosphere through burning fossil fuels • This causes climate change and alters average temperatures disrupting ecosystems • Deforestation reduces the amount of photosynthesis needed for absorption of carbon dioxide

The Nitrogen Cycle • The atmosphere is composed of 78% nitrogen gas, N 2 but plants cannot use nitrogen in this form, it must be supplied in other forms: • ammonium ion (NH 4+) • nitrate ion (NO 3 -)

Making Nitrogen Useable Nitrogen Fixation • The process of converting N 2 gas into usable sources, such as ammonium (NH 4+) • This accomplished by bacteria or lightning. • Without bacteria, movement of nitrogen would almost STOP completely! Denitrifying Bacteria: • The process of converting nitrates (NO 3 -) back into nitrogen gas (N 2)


Class/Homework • WS “Cycling of Matter” • Pg. 51 #5, 7 - 10
 Matter cycling in ecosystems
Matter cycling in ecosystems Energy flow and chemical recycling in ecosystems
Energy flow and chemical recycling in ecosystems Cycling of matter in an ecosystem
Cycling of matter in an ecosystem Section 3 cycling of matter answer key
Section 3 cycling of matter answer key Section 1 organisms and their relationships
Section 1 organisms and their relationships Principles of ecology section 3 cycling of matter
Principles of ecology section 3 cycling of matter Section 1 organisms and their relationships
Section 1 organisms and their relationships Cycling of matter definition biology
Cycling of matter definition biology Phosphorus cycle
Phosphorus cycle Provides practically all the energy for ecosystems
Provides practically all the energy for ecosystems Name all the lines
Name all the lines A bird stalks kills then eats
A bird stalks kills then eats Noaa hysplit
Noaa hysplit Quantitative research about cycling
Quantitative research about cycling Tpn tapering guidelines
Tpn tapering guidelines Energy flow and material cycling in ecosystem
Energy flow and material cycling in ecosystem Cycling event sponsorship proposal
Cycling event sponsorship proposal Road cycling 101
Road cycling 101 Lateral cycling consumer behavior
Lateral cycling consumer behavior