CutnellJohnson Physics 7 th edition Classroom Response System

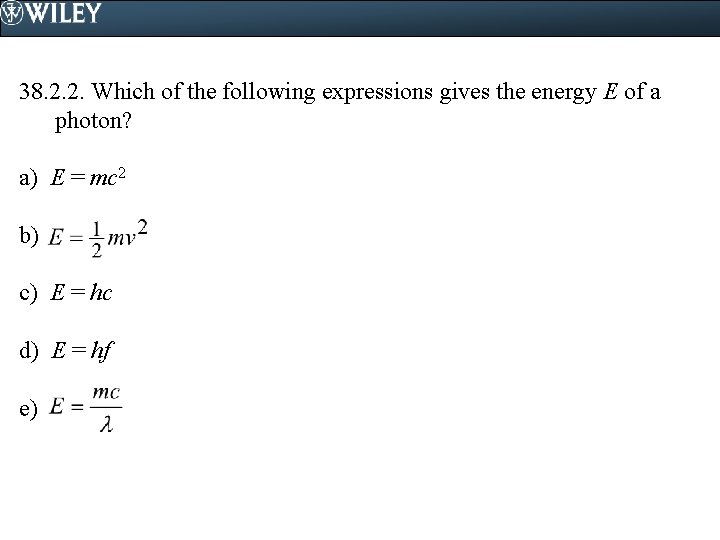
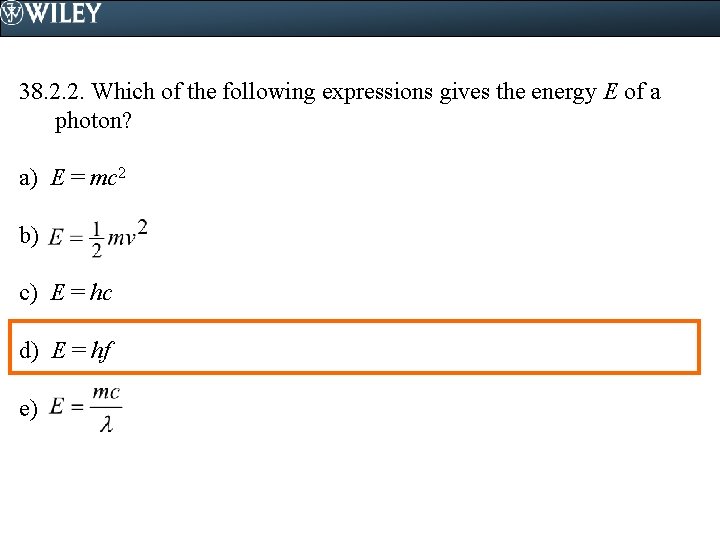




































- Slides: 45

Cutnell/Johnson Physics 7 th edition Classroom Response System Questions Chapter 38 Photon and Matter Waves Reading Quiz Questions

38. 2. 1. What is the name given to the particle-like entities that compose electromagnetic waves? a) phonons b) photons c) mesons d) leptons e) electromagnons

38. 2. 1. What is the name given to the particle-like entities that compose electromagnetic waves? a) phonons b) photons c) mesons d) leptons e) electromagnons
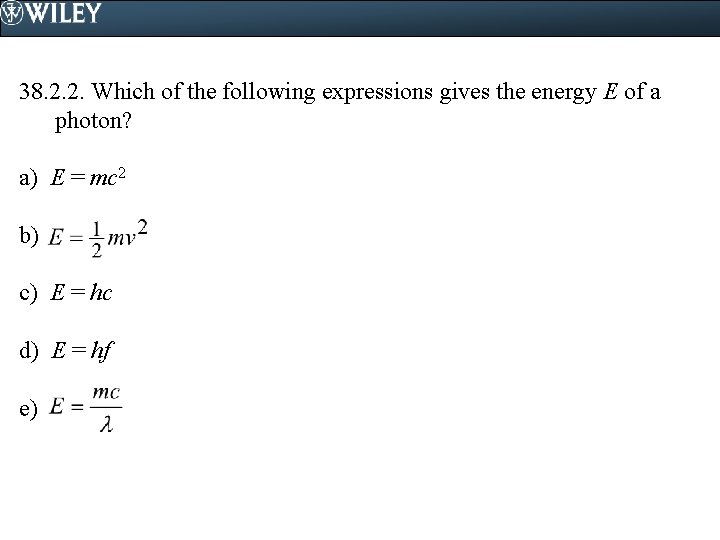
38. 2. 2. Which of the following expressions gives the energy E of a photon? a) E = mc 2 b) c) E = hc d) E = hf e)
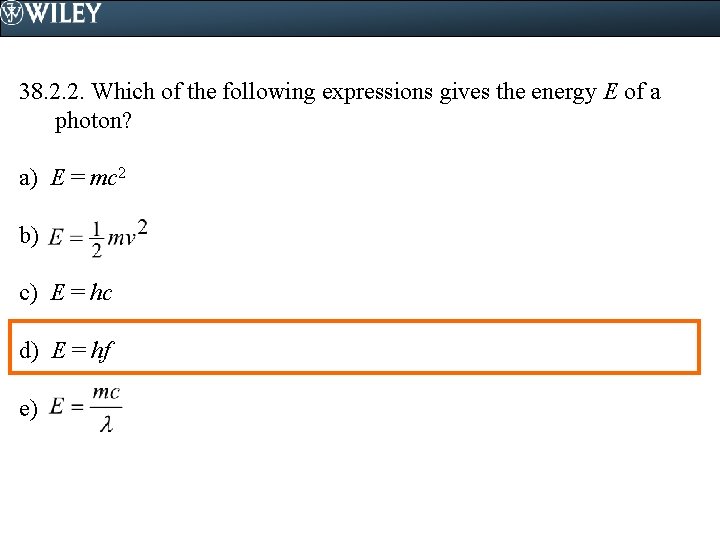
38. 2. 2. Which of the following expressions gives the energy E of a photon? a) E = mc 2 b) c) E = hc d) E = hf e)

38. 2. 3. On what does the energy of a photon depend? a) the speed of the photon b) the mass of the photon c) the size of the photon d) the frequency of the photon

38. 2. 3. On what does the energy of a photon depend? a) the speed of the photon b) the mass of the photon c) the size of the photon d) the frequency of the photon

38. 2. 4. How does the energy of a “red”photon compare with that of a “blue” photon? a) It is less. b) It is the same. c) It is more.

38. 2. 4. How does the energy of a “red”photon compare with that of a “blue” photon? a) It is less. b) It is the same. c) It is more.

38. 2. 5. How does the energy of light with a shorter wavelength compare with that of light with a longer wavelength? a) It has less energy. b) It has the same energy. c) It has more energy.

38. 2. 5. How does the energy of light with a shorter wavelength compare with that of light with a longer wavelength? a) It has less energy. b) It has the same energy. c) It has more energy.

38. 3. 1. Which one of the following experiments provides evidence that light consists of particle-like entities? a) Young’s double slit b) photoelectric effect c) blackbody radiation d) Rutherford scattering e) crossed polarizers

38. 3. 1. Which one of the following experiments provides evidence that light consists of particle-like entities? a) Young’s double slit b) photoelectric effect c) blackbody radiation d) Rutherford scattering e) crossed polarizers

38. 3. 2. What is the term used for the minimum work needed to eject an electron from a metal surface? a) photoelectric function b) photon energy c) work function d) Fermi energy e) Coulomb potential

38. 3. 2. What is the term used for the minimum work needed to eject an electron from a metal surface? a) photoelectric function b) photon energy c) work function d) Fermi energy e) Coulomb potential

38. 3. 3. What did the photoelectric effect experiment demonstrate? a) Nothing, it was a complete failure. b) Photons have mass. c) The speed of light is the “speed limit” of the Universe. d) Light can behave like particles. e) Electrons can behave like waves.

38. 3. 3. What did the photoelectric effect experiment demonstrate? a) Nothing, it was a complete failure. b) Photons have mass. c) The speed of light is the “speed limit” of the Universe. d) Light can behave like particles. e) Electrons can behave like waves.

38. 4. 1. For which of the following physical discoveries is Arthur Compton credited? a) The circumference of an electron’s orbit in an atom is an integer multiple of the electron’s wavelength. b) The positive charge within an atom is concentrated within a very small volume within an atom. c) A low pressure monatomic gas can be made to emit electromagnetic waves viewed as a series of specific bright fringes. d) The scattering of x-rays by electrons in graphite further demonstrates that light can exhibit particle-like characteristics. e) The more precisely the position of an electron is determined, the less precisely the momentum of the electron is known at a given time.

38. 4. 1. For which of the following physical discoveries is Arthur Compton credited? a) The circumference of an electron’s orbit in an atom is an integer multiple of the electron’s wavelength. b) The positive charge within an atom is concentrated within a very small volume within an atom. c) A low pressure monatomic gas can be made to emit electromagnetic waves viewed as a series of specific bright fringes. d) The scattering of x-rays by electrons in graphite further demonstrates that light can exhibit particle-like characteristics. e) The more precisely the position of an electron is determined, the less precisely the momentum of the electron is known at a given time.

38. 4. 2. Complete the following statement: When an x-ray photon collides with an electron at rest, a) the electron turns into a photon. b) the magnitude of the scattered photon is the same as the magnitude of the incident x-ray photon, but the direction of the momentum is altered. c) the frequency of the scattered photon is less than the frequency of the incident x-ray photon. d) the electron absorbs the photon and becomes a proton. e) the energy of the x-ray photon is completely absorbed by the electron.

38. 4. 2. Complete the following statement: When an x-ray photon collides with an electron at rest, a) the electron turns into a photon. b) the magnitude of the scattered photon is the same as the magnitude of the incident x-ray photon, but the direction of the momentum is altered. c) the frequency of the scattered photon is less than the frequency of the incident x-ray photon. d) the electron absorbs the photon and becomes a proton. e) the energy of the x-ray photon is completely absorbed by the electron.

38. 4. 3. When the Compton shift was measured, what was demonstrated? a) Photons have momentum. b) Photons have energy. c) Photons behave like waves. d) Photons have no mass. e) Electrons have no mass.

38. 4. 3. When the Compton shift was measured, what was demonstrated? a) Photons have momentum. b) Photons have energy. c) Photons behave like waves. d) Photons have no mass. e) Electrons have no mass.

38. 5. 1. What would one observe on an observation screen if a single photon was allowed to pass through one of the slits in a double slit experiment? a) You would see a faint diffraction pattern. b) You would see a single bright point. c) You would see a central band, but no other bands. d) You would see two bright bands. e) You would see a faint interference pattern.

38. 5. 1. What would one observe on an observation screen if a single photon was allowed to pass through one of the slits in a double slit experiment? a) You would see a faint diffraction pattern. b) You would see a single bright point. c) You would see a central band, but no other bands. d) You would see two bright bands. e) You would see a faint interference pattern.

38. 6. 1. When a beam of electrons is directed at a suitably narrow pair of slits, what is observed at a screen behind the double slit? a) an image of the double slit b) two bright regions with extended, fuzzy edges c) one bright region with intensity decreasing exponentially on each side d) one sharp bright region e) alternating bright and dark regions

38. 6. 1. When a beam of electrons is directed at a suitably narrow pair of slits, what is observed at a screen behind the double slit? a) an image of the double slit b) two bright regions with extended, fuzzy edges c) one bright region with intensity decreasing exponentially on each side d) one sharp bright region e) alternating bright and dark regions

38. 6. 2. Which one of the following statements best explains what is meant by the “dual nature” of the electron? a) An electron may act with either particle-like or wave-like characteristics. b) An electron may be either in a quasi-free state as in a metal or in a tightlybound state deep within an atom. c) An electron can be transmitted in a beam as in a television or through a wire. d) An electron plays a role in the production of both magnetic and electric fields. e) An electron can travel at very small velocities or be at rest. It’s motion adequately described by Newton’s laws of motion. The electron can also travel at speeds close to the speed of light; and its motion is described by relativistic laws of motion.

38. 6. 2. Which one of the following statements best explains what is meant by the “dual nature” of the electron? a) An electron may act with either particle-like or wave-like characteristics. b) An electron may be either in a quasi-free state as in a metal or in a tightlybound state deep within an atom. c) An electron can be transmitted in a beam as in a television or through a wire. d) An electron plays a role in the production of both magnetic and electric fields. e) An electron can travel at very small velocities or be at rest. It’s motion adequately described by Newton’s laws of motion. The electron can also travel at speeds close to the speed of light; and its motion is described by relativistic laws of motion.

38. 6. 3. Which one of the following scientists made the suggestion that since light waves can exhibit particle-like properties, that particles should exhibit wave-like properties? a) de Broglie b) Planck c) Heisenberg d) Compton e) Einstein

38. 6. 3. Which one of the following scientists made the suggestion that since light waves can exhibit particle-like properties, that particles should exhibit wave-like properties? a) de Broglie b) Planck c) Heisenberg d) Compton e) Einstein

38. 7. 1. The square of what parameter indicates the probability of locating a particle within a region of space? a) momentum b) wave function c) wavelength d) energy e) spin

38. 7. 1. The square of what parameter indicates the probability of locating a particle within a region of space? a) momentum b) wave function c) wavelength d) energy e) spin

38. 7. 2. What does the Schrödinger equation describe? a) string waves b) matter waves c) light waves d) electromagnetic forces e) the gravitational force

38. 7. 2. What does the Schrödinger equation describe? a) string waves b) matter waves c) light waves d) electromagnetic forces e) the gravitational force

38. 8. 1. Which branch of physics emerged from the work of Schrödinger and Heisenberg? a) plasma physics b) nuclear physics c) quantum electrodynamics d) astrophysics e) quantum mechanics

38. 8. 1. Which branch of physics emerged from the work of Schrödinger and Heisenberg? a) plasma physics b) nuclear physics c) quantum electrodynamics d) astrophysics e) quantum mechanics

38. 8. 2. Complete the following statement: The shorter the lifetime of a particle in a given energy state, a) the more precise is the measurement of the energy of the particle. b) the greater the energy of that state. c) the greater is the uncertainty in the energy of that state. d) the more precise is the measurement of the lifetime. e) the more likely the particle will gain energy.

38. 8. 2. Complete the following statement: The shorter the lifetime of a particle in a given energy state, a) the more precise is the measurement of the energy of the particle. b) the greater the energy of that state. c) the greater is the uncertainty in the energy of that state. d) the more precise is the measurement of the lifetime. e) the more likely the particle will gain energy.

38. 8. 3. Which one of the following statements is true according to the Heisenberg uncertainty principle? a) Two electrons within an atom cannot have the same quantum numbers. b) You can never measure the location of a particle. c) Quantum mechanics is only a theory. d) It is impossible to know both the position and momentum of a particle at the same time. e) You can never make a measurement that is more precise than h/2.

38. 8. 3. Which one of the following statements is true according to the Heisenberg uncertainty principle? a) Two electrons within an atom cannot have the same quantum numbers. b) You can never measure the location of a particle. c) Quantum mechanics is only a theory. d) It is impossible to know both the position and momentum of a particle at the same time. e) You can never make a measurement that is more precise than h/2.

38. 9. 1. What is barrier tunneling? a) This occurs when photons pass through a solid barrier. b) This occurs when particles make a hole through a solid barrier. c) This occurs when photons make a hole through a energy barrier. d) This occurs when particles pass through an energy barrier. e) This occurs when photons refract at a surface.

38. 9. 1. What is barrier tunneling? a) This occurs when photons pass through a solid barrier. b) This occurs when particles make a hole through a solid barrier. c) This occurs when photons make a hole through a energy barrier. d) This occurs when particles pass through an energy barrier. e) This occurs when photons refract at a surface.

38. 9. 2. Which of the following does not affect the probability that a particle will penetrate an energy barrier? a) the potential energy of the barrier b) the kinetic energy of the particle c) the potential energy of the particle d) the total energy of the particle e) All of the above choices affect the probability.

38. 9. 2. Which of the following does not affect the probability that a particle will penetrate an energy barrier? a) the potential energy of the barrier b) the kinetic energy of the particle c) the potential energy of the particle d) the total energy of the particle e) All of the above choices affect the probability.