Culturing experiments to determine the nutrient requirement for




- Slides: 20

Culturing experiments to determine the nutrient requirement for exopolymer production in Lentisphaera araneosa By Connie Lee PI: Stephen Giovannoni Mentor: Amy Carter Department of Microbiology , OSU HHMI Summer Program 2011

Significance �Marine transparent exopolysaccharides (TEP) �Marine snow �Recycle organic compounds �Movement of organic matter �More efficient culture medium �Foundation for further investigations �Symbiotic relationship �Ecological role �Potential use in industries

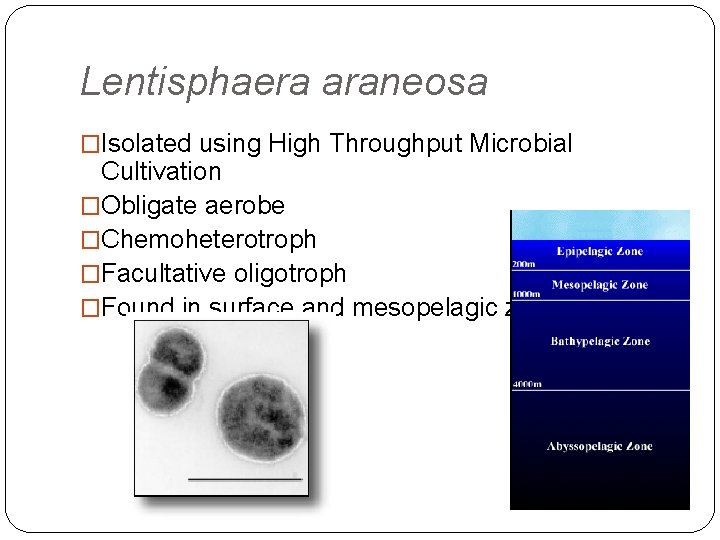
Lentisphaera araneosa �Isolated using High Throughput Microbial Cultivation �Obligate aerobe �Chemoheterotroph �Facultative oligotroph �Found in surface and mesopelagic zones

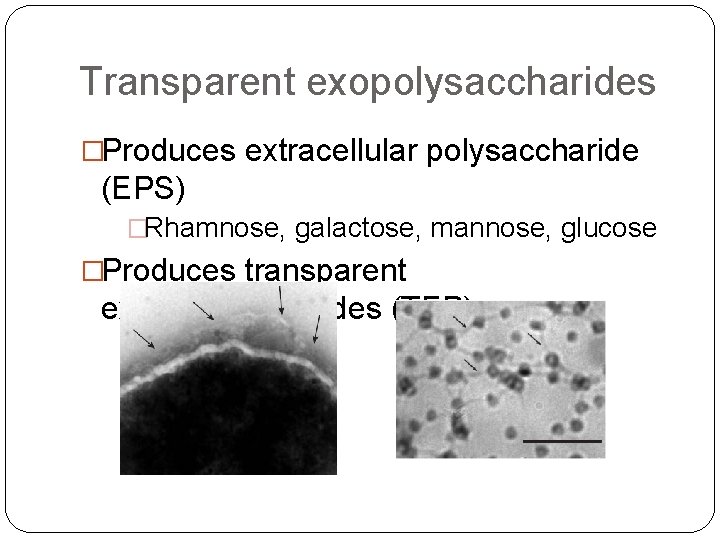
Transparent exopolysaccharides �Produces extracellular polysaccharide (EPS) �Rhamnose, galactose, mannose, glucose �Produces transparent exopolysaccharides (TEP)

Cell Growth �Grows in medium composed of various carbon sources �In low nutrient heterotrophic medium �In artificial seawater �Optimum growth temperature at 16 - 20 degree Celsius �Fast growth rate Composition of current medium • • Glycine Methionine Serine Pyruvate Taurine Oxaloacetic acid Glucose

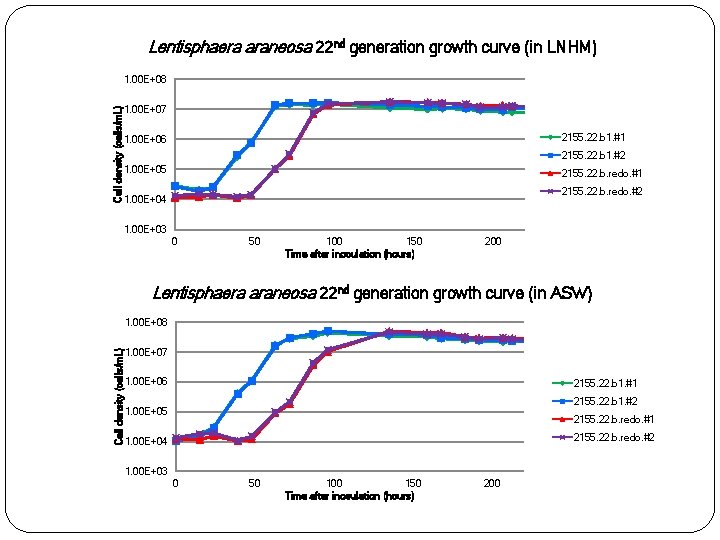
Lentisphaera araneosa 22 nd generation growth curve (in LNHM) Cell density (cells/m. L) 1. 00 E+08 1. 00 E+07 2155. 22. b 1. #1 1. 00 E+06 2155. 22. b 1. #2 1. 00 E+05 2155. 22. b. redo. #1 2155. 22. b. redo. #2 1. 00 E+04 1. 00 E+03 0 50 100 150 Time after inoculation (hours) 200 Lentisphaera araneosa 22 nd generation growth curve (in ASW) Cell density (cells/m. L) 1. 00 E+08 1. 00 E+07 1. 00 E+06 2155. 22. b 1. #1 2155. 22. b 1. #2 1. 00 E+05 2155. 22. b. redo. #1 2155. 22. b. redo. #2 1. 00 E+04 1. 00 E+03 0 50 100 150 Time after inoculation (hours) 200

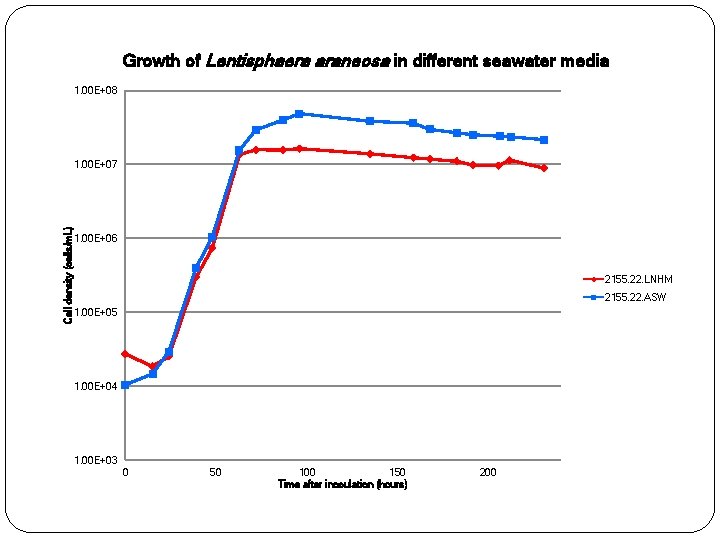
Growth of Lentisphaera araneosa in different seawater media 1. 00 E+08 Cell density (cells/m. L) 1. 00 E+07 1. 00 E+06 2155. 22. LNHM 2155. 22. ASW 1. 00 E+05 1. 00 E+04 1. 00 E+03 0 50 100 150 Time after inoculation (hours) 200

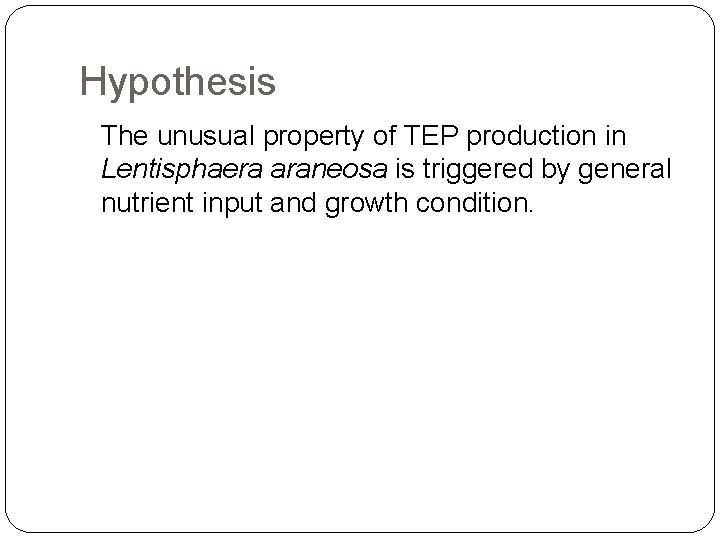
Hypothesis The unusual property of TEP production in Lentisphaera araneosa is triggered by general nutrient input and growth condition.

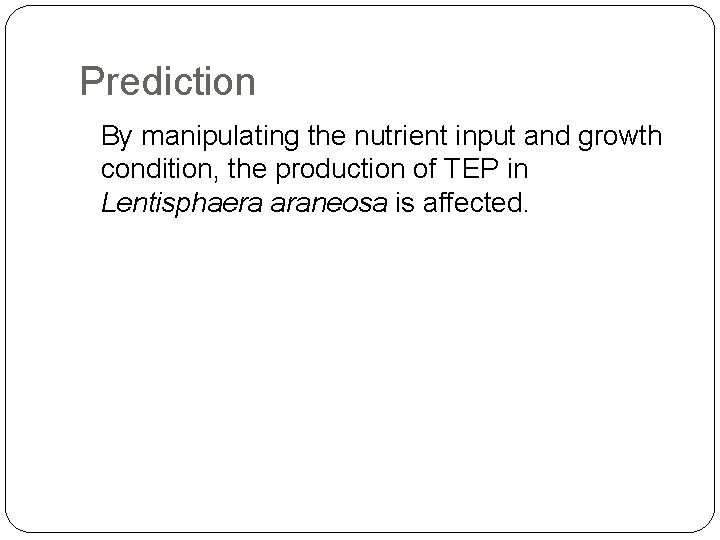
Prediction By manipulating the nutrient input and growth condition, the production of TEP in Lentisphaera araneosa is affected.

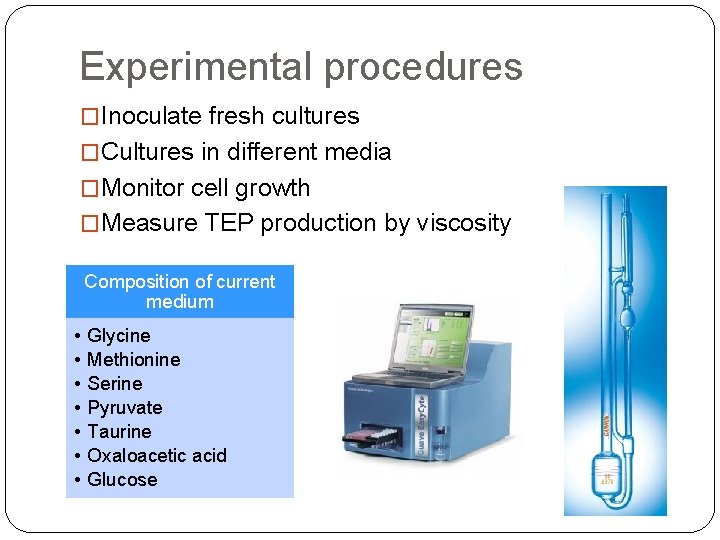
Experimental procedures �Inoculate fresh cultures �Cultures in different media �Monitor cell growth �Measure TEP production by viscosity Composition of current medium • • Glycine Methionine Serine Pyruvate Taurine Oxaloacetic acid Glucose

Oxygen requirement of Lentisphaera araneosa 1. 00 E+08 Cell density (cells/m. L) 1. 00 E+07 1. 00 E+06 100 m. L culture 150 m. L culture 1. 00 E+05 200 m. L culture 1. 00 E+04 1. 00 E+03 0 50 100 150 Time after inoculation (hours) 200 250

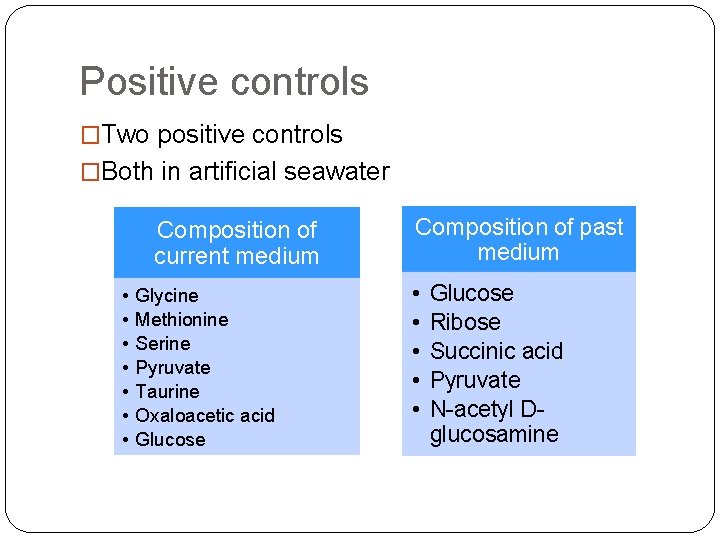
Positive controls �Two positive controls �Both in artificial seawater Composition of current medium • • Glycine Methionine Serine Pyruvate Taurine Oxaloacetic acid Glucose Composition of past medium • • • Glucose Ribose Succinic acid Pyruvate N-acetyl Dglucosamine

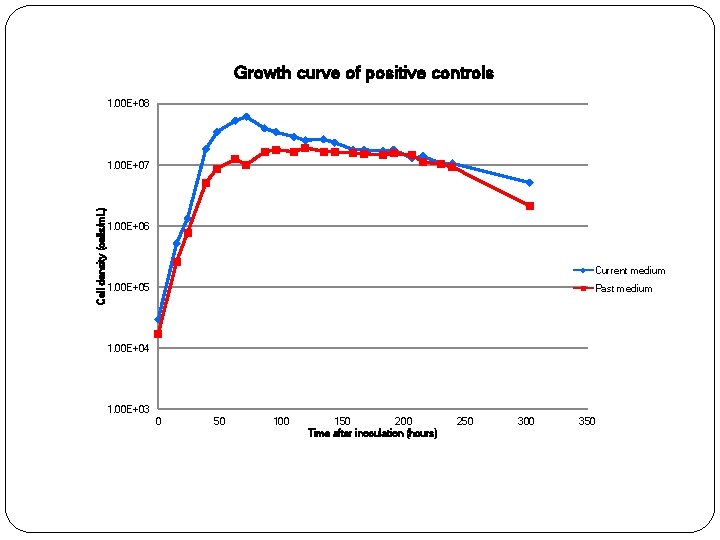
Growth curve of positive controls 1. 00 E+08 Cell density (cells/m. L) 1. 00 E+07 1. 00 E+06 Current medium 1. 00 E+05 Past medium 1. 00 E+04 1. 00 E+03 0 50 100 150 200 Time after inoculation (hours) 250 300 350

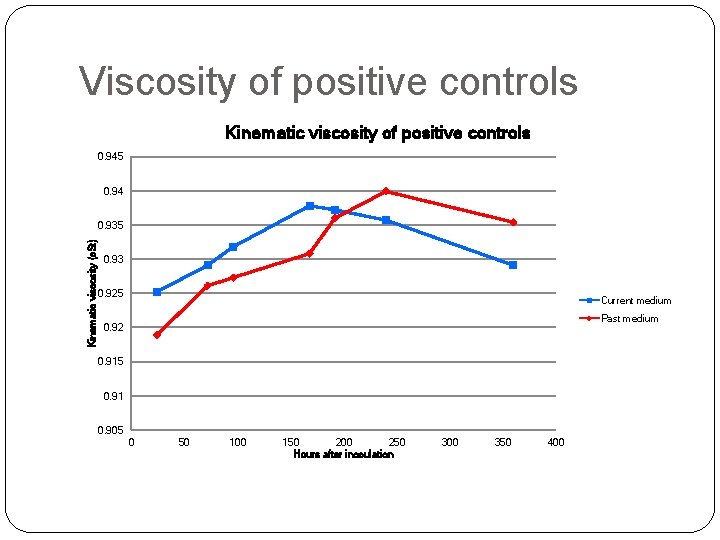
Viscosity of positive controls Kinematic viscosity of positive controls 0. 945 0. 94 Kinematic viscosity (c. St) 0. 935 0. 93 0. 925 Current medium Past medium 0. 92 0. 915 0. 91 0. 905 0 50 100 150 200 250 Hours after inoculation 300 350 400

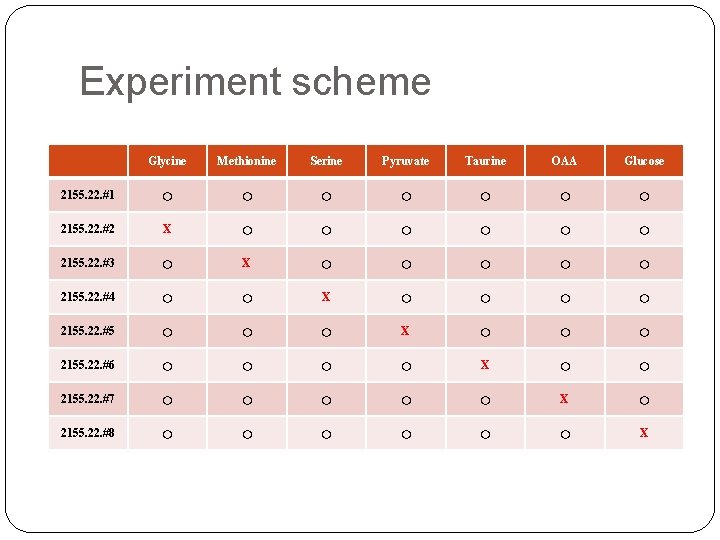
Experiment scheme Glycine Methionine Serine Pyruvate Taurine OAA Glucose 2155. 22. #1 ○ ○ ○ ○ 2155. 22. #2 X ○ ○ ○ 2155. 22. #3 ○ X ○ ○ ○ 2155. 22. #4 ○ ○ X ○ ○ 2155. 22. #5 ○ ○ ○ X ○ ○ ○ 2155. 22. #6 ○ ○ X ○ ○ 2155. 22. #7 ○ ○ ○ X ○ 2155. 22. #8 ○ ○ ○ X

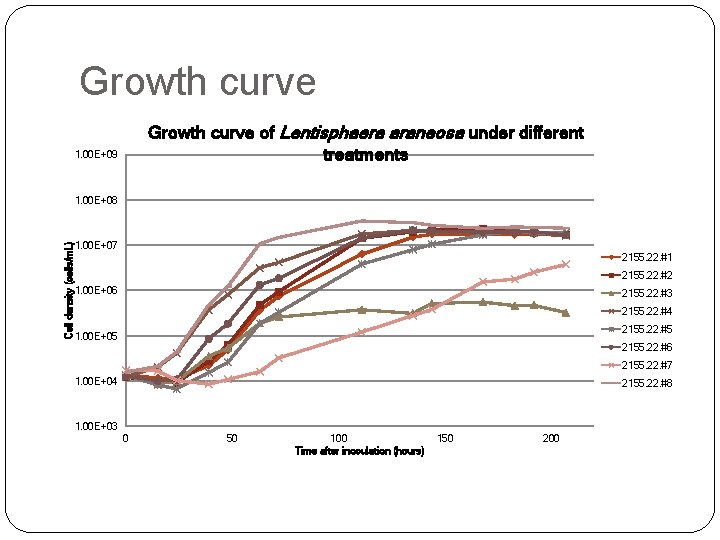
Cell density (cells/m. L) Growth curve Time after inoculation (hours)

Viscosity Kinematic viscosity of Lentisphaera araneosa under different treatments 0. 944 0. 942 Kinematic viscosity (c. St) 0. 94 0. 938 2155. 22. #1 0. 936 2155. 22. #2 0. 934 2155. 22. #3 0. 932 2155. 22. #4 2155. 22. #5 0. 93 2155. 22. #6 0. 928 2155. 22. #7 0. 926 2155. 22. #8 0. 924 0. 922 0 50 100 Time after inoculation (hours) 150 200

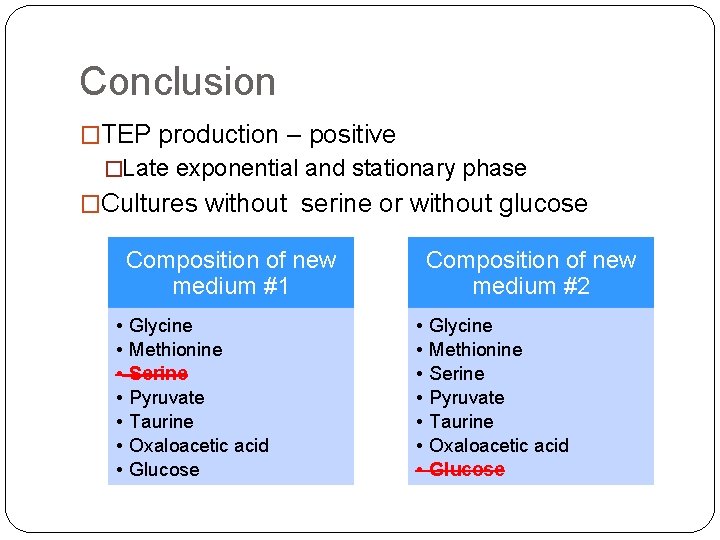
Conclusion �TEP production – positive �Late exponential and stationary phase �Cultures without serine or without glucose Composition of new medium #1 • • Glycine Methionine Serine Pyruvate Taurine Oxaloacetic acid Glucose Composition of new medium #2 • • Glycine Methionine Serine Pyruvate Taurine Oxaloacetic acid Glucose

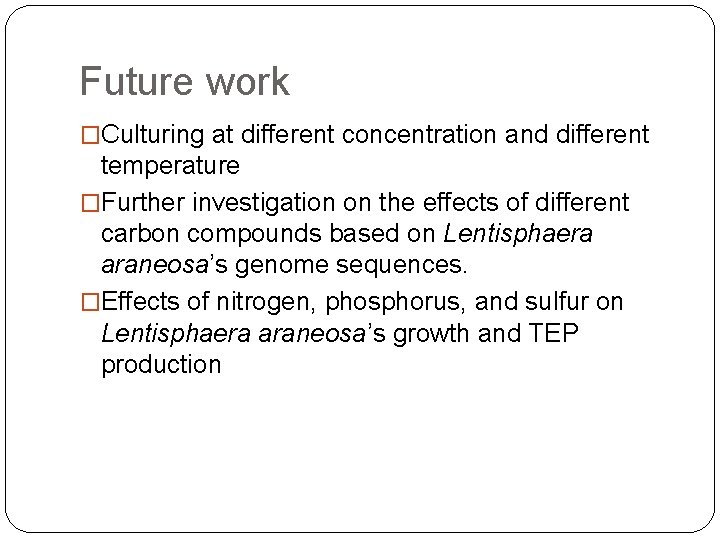
Future work �Culturing at different concentration and different temperature �Further investigation on the effects of different carbon compounds based on Lentisphaera araneosa’s genome sequences. �Effects of nitrogen, phosphorus, and sulfur on Lentisphaera araneosa’s growth and TEP production

Acknowledgement � Dr. Stephen Giovannoni � Amy Carter � The Stephen Giovannoni Lab �Kevin Vergin �Dr. Jang-Cheon Cho �Paul Carini �Dr. Yanlin Zhao � Dr. Kevin Ahern � The Howard Hughes Medical Institute � URISC � Oregon State University Honors College � Cripps scholarships