Culture Types Callus Culture Cellsuspension Culture Tissue or











- Slides: 34

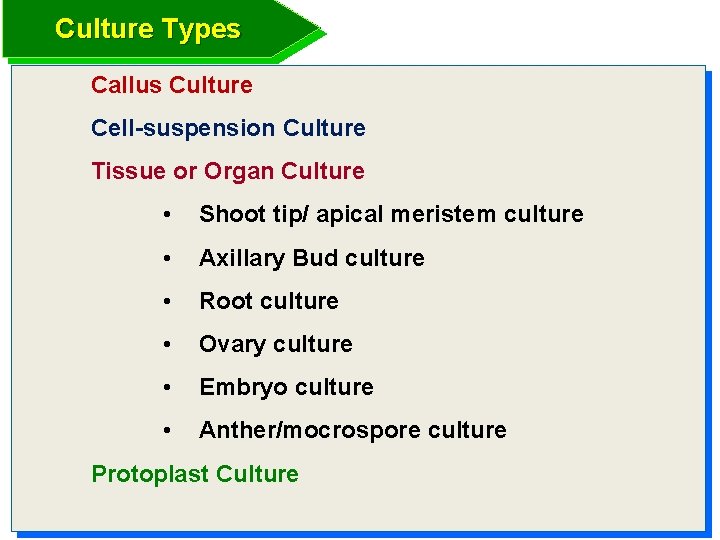
Culture Types Callus Culture Cell-suspension Culture Tissue or Organ Culture • Shoot tip/ apical meristem culture • Axillary Bud culture • Root culture • Ovary culture • Embryo culture • Anther/mocrospore culture Protoplast Culture

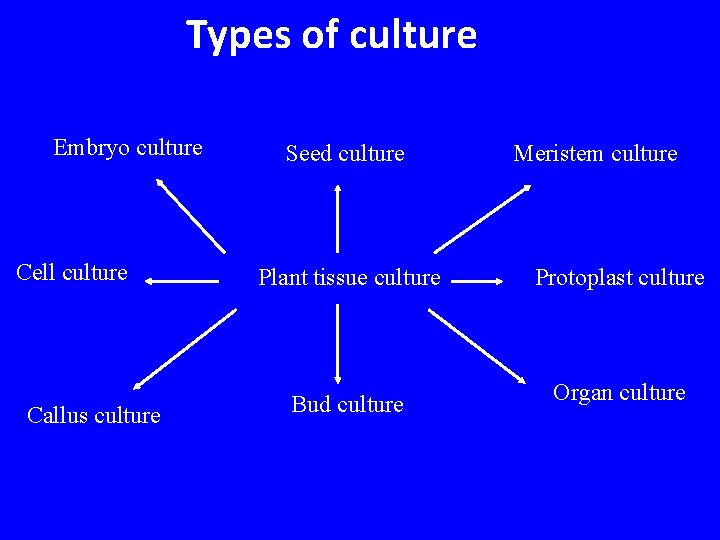
Types of culture Embryo culture Cell culture Callus culture Seed culture Meristem culture Plant tissue culture Protoplast culture Bud culture Organ culture

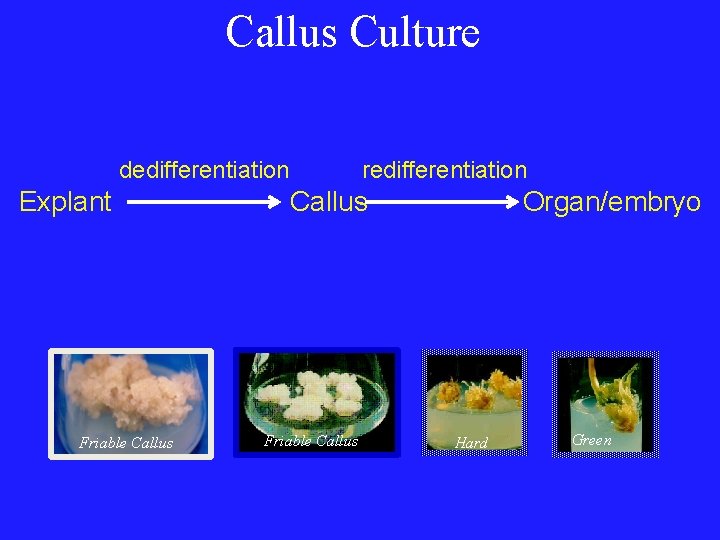
Callus Culture dedifferentiation Explant Friable Callus redifferentiation Callus Friable Callus Organ/embryo Hard Green

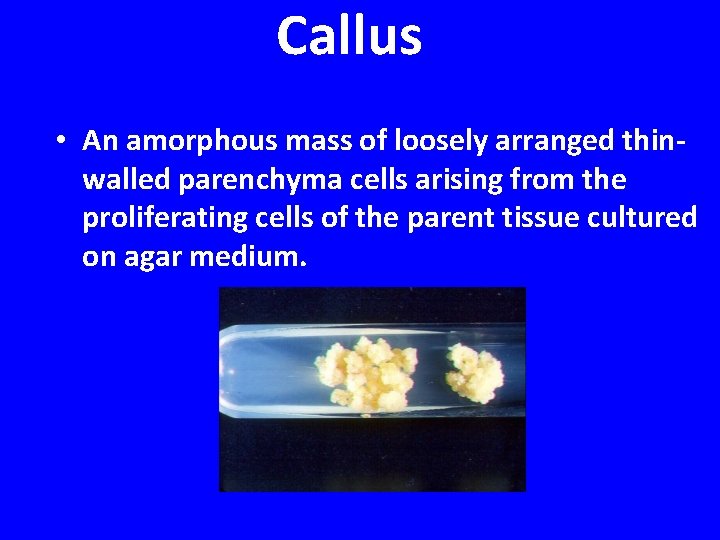
Callus • An amorphous mass of loosely arranged thinwalled parenchyma cells arising from the proliferating cells of the parent tissue cultured on agar medium.

Cellular totipotency • Cytodifferentiation – Cell differentiation, mainly emphasis on vascular differentiation, tracheary element differentiation, etc. • Dedifferentiation – The phenomenon of mature cells reverting to a meristematic state and forming undifferentiated callus tissue. • Redifferentiation – The ability of the component cells of the callus to differentiate into a whole plant or organ.

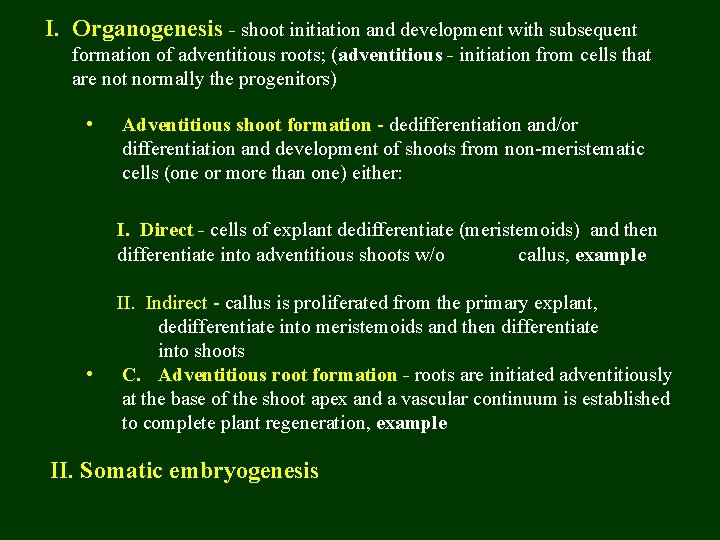
I. Organogenesis - shoot initiation and development with subsequent formation of adventitious roots; (adventitious - initiation from cells that are not normally the progenitors) • Adventitious shoot formation - dedifferentiation and/or differentiation and development of shoots from non-meristematic cells (one or more than one) either: I. Direct - cells of explant dedifferentiate (meristemoids) and then differentiate into adventitious shoots w/o callus, example • II. Indirect - callus is proliferated from the primary explant, dedifferentiate into meristemoids and then differentiate into shoots C. Adventitious root formation - roots are initiated adventitiously at the base of the shoot apex and a vascular continuum is established to complete plant regeneration, example II. Somatic embryogenesis

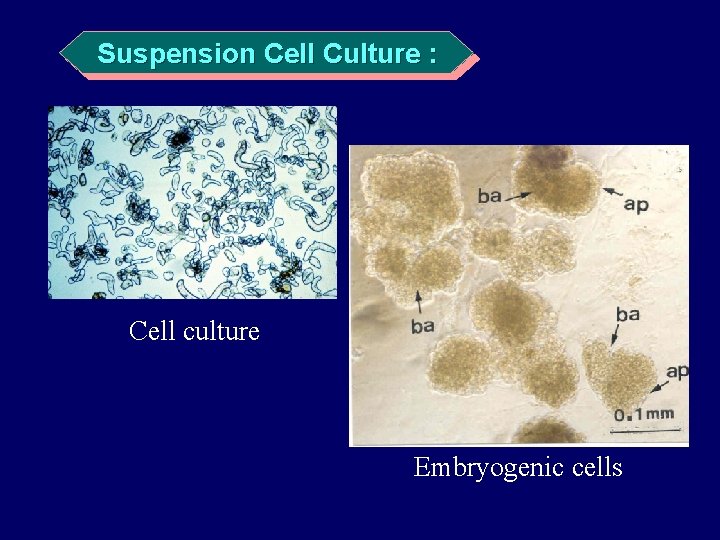
Suspension Cell Culture : A type of culture in which cells and/or clumps of cells grow and multiply while suspended in a liquid medium • Rapidly dividing • Homogenous cells or cell aggregates • Suspended in a liquid medium • Cultured to produce a “cell line”。

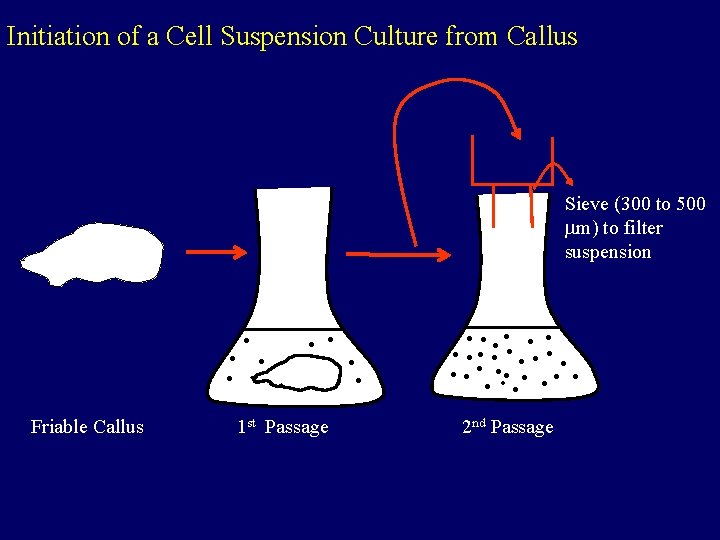
Initiation of a Cell Suspension Culture from Callus Sieve (300 to 500 m) to filter suspension Friable Callus 1 st Passage 2 nd Passage

Suspension Cell Culture : Cell culture Embryogenic cells

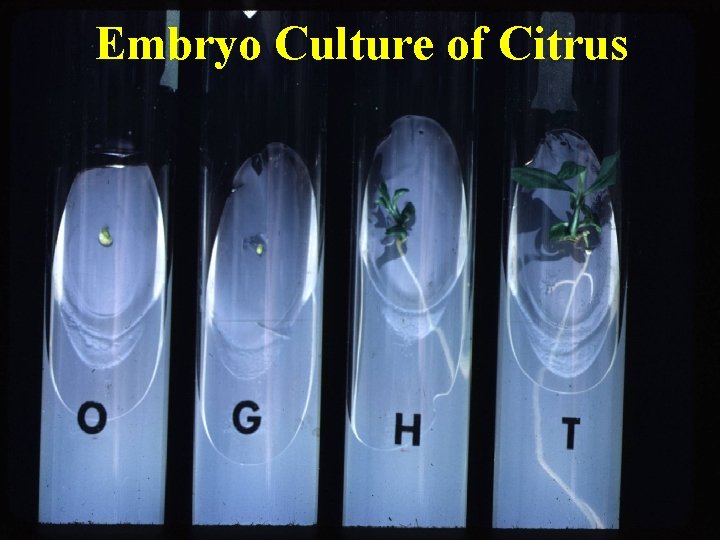
Embryo culture • Mature embryo culture – Seed dormancy (ripe seeds) • Immature embryo / embryo rescue – To avoid embryo abortion (Hybrid embryo)

Application of embryo culture • Prevention of embryo abortion in wide crosses. • Production of haploids • Overcoming seed dormancy • Shortening of breeding cycle

Embryo Culture of Citrus

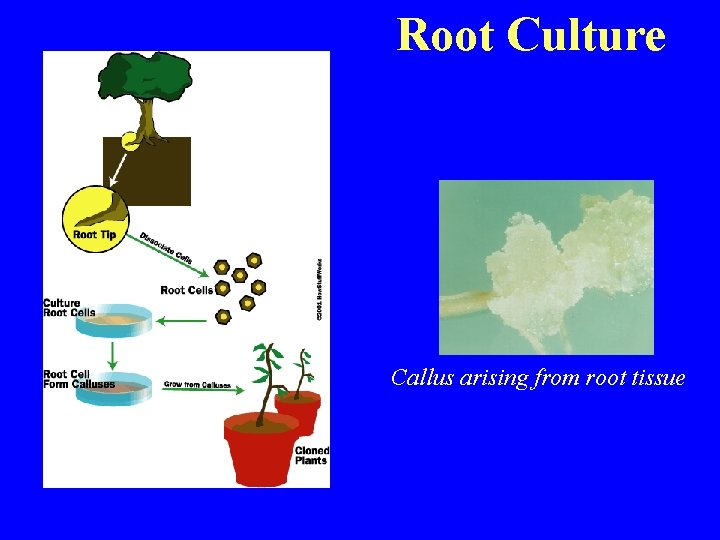
Root Culture Callus arising from root tissue

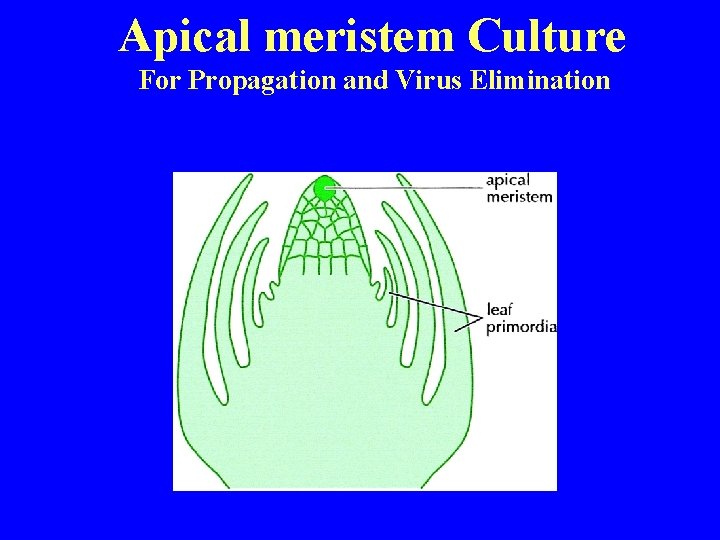
Apical meristem Culture For Propagation and Virus Elimination

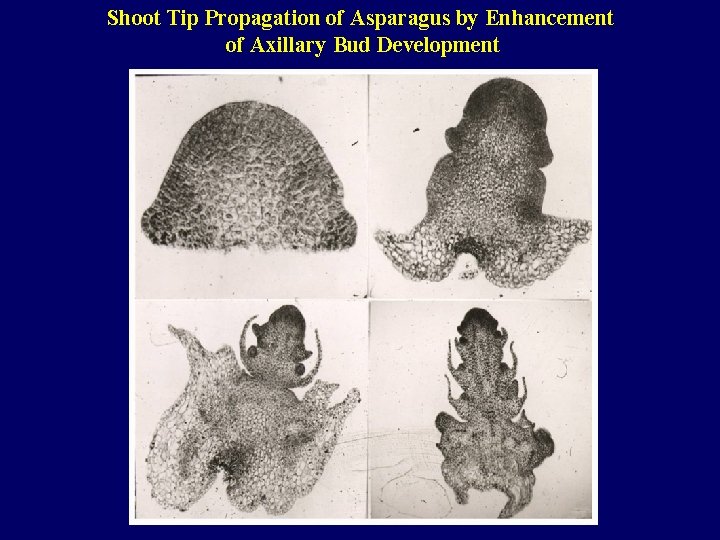
Shoot Tip Propagation of Asparagus by Enhancement of Axillary Bud Development

Anther culture 1964 Guha & Maheshwari Anther culture ---> haploid plant ( Datura ) 1968 Niizeki & Oono : (Japan) Haploid plant of rice * Started for plant breeding

Anther and microspore culture

Anther culture • Culturing methods – anther culture – easiest and simplest – protocol for tobacco anther culture • (aseptically) detach anther from tobacco filament • float anther on a liquid (MS-type) culture medium

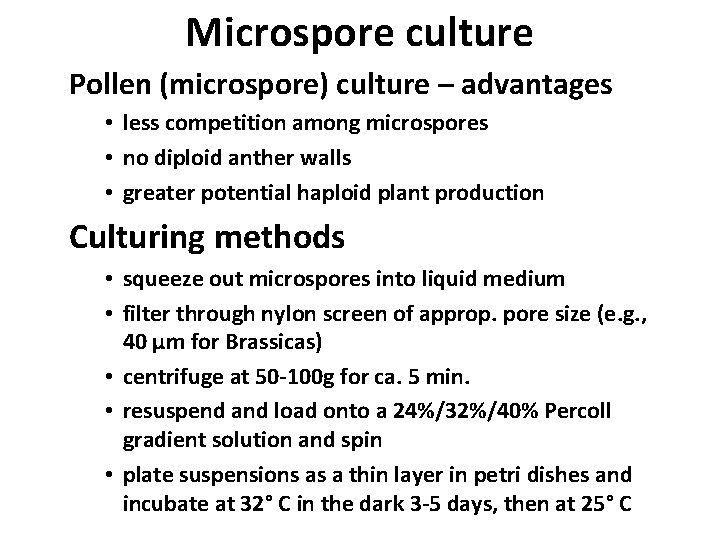
Microspore culture • Culturing methods – pollen (microspore) culture – advantages • less competition among microspores • no diploid anther walls • greater potential haploid plant production

Microspore culture Pollen (microspore) culture – advantages • less competition among microspores • no diploid anther walls • greater potential haploid plant production Culturing methods • squeeze out microspores into liquid medium • filter through nylon screen of approp. pore size (e. g. , 40 μm for Brassicas) • centrifuge at 50 -100 g for ca. 5 min. • resuspend and load onto a 24%/32%/40% Percoll gradient solution and spin • plate suspensions as a thin layer in petri dishes and incubate at 32° C in the dark 3 -5 days, then at 25° C

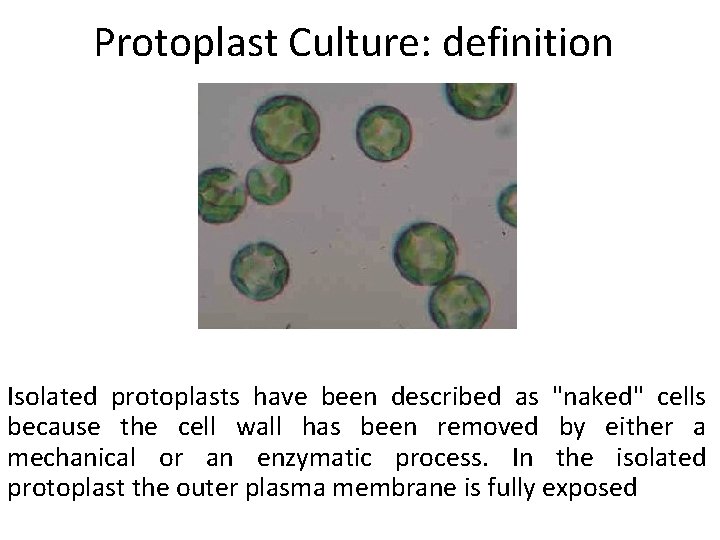
Protoplast Culture: definition Isolated protoplasts have been described as "naked" cells because the cell wall has been removed by either a mechanical or an enzymatic process. In the isolated protoplast the outer plasma membrane is fully exposed

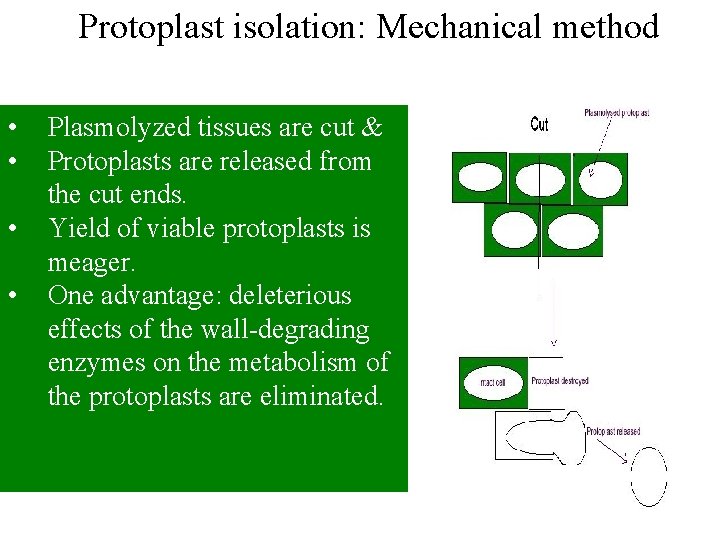
Protoplast isolation: Mechanical method • • Plasmolyzed tissues are cut & Protoplasts are released from the cut ends. Yield of viable protoplasts is meager. One advantage: deleterious effects of the wall-degrading enzymes on the metabolism of the protoplasts are eliminated.

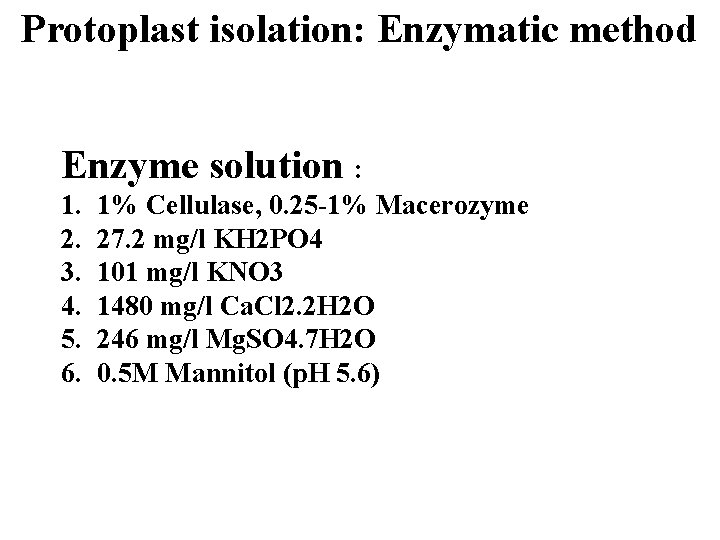
Protoplast isolation: Enzymatic method Enzyme solution : 1. 2. 3. 4. 5. 6. 1% Cellulase, 0. 25 -1% Macerozyme 27. 2 mg/l KH 2 PO 4 101 mg/l KNO 3 1480 mg/l Ca. Cl 2. 2 H 2 O 246 mg/l Mg. SO 4. 7 H 2 O 0. 5 M Mannitol (p. H 5. 6)

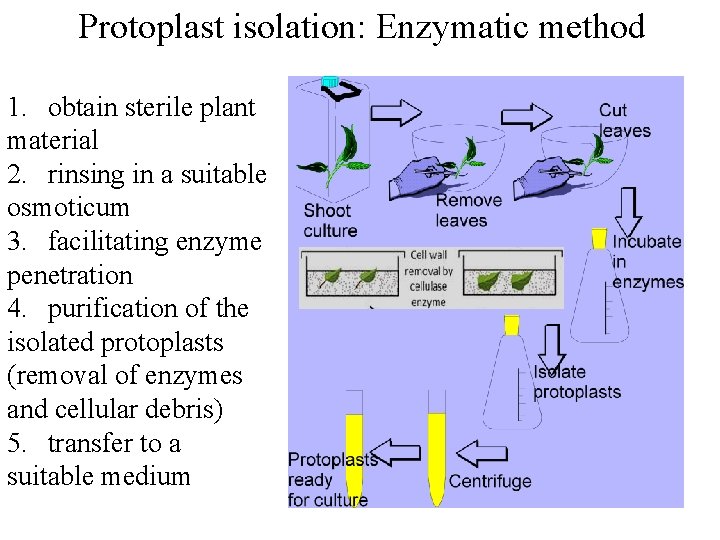
Protoplast isolation: Enzymatic method 1. obtain sterile plant material 2. rinsing in a suitable osmoticum 3. facilitating enzyme penetration 4. purification of the isolated protoplasts (removal of enzymes and cellular debris) 5. transfer to a suitable medium

Protoplasts Fusion Protoplast Transformation wall synthesis Single cell systems

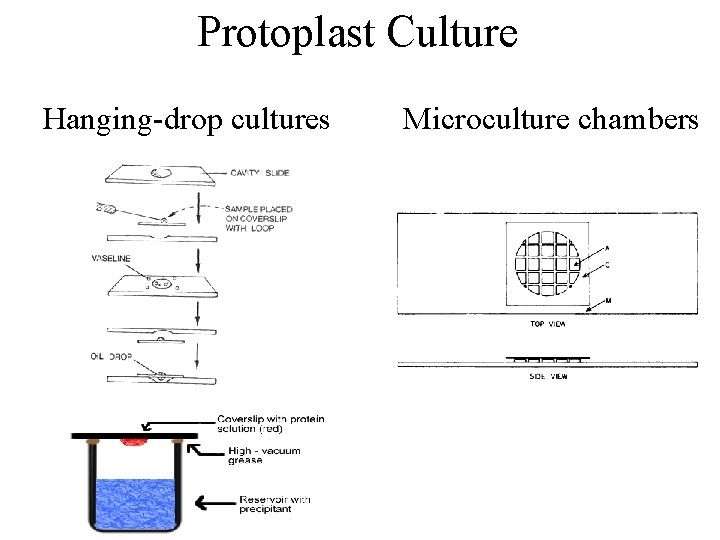
Protoplast Culture Protoplasts can been cultured in several ways: 1. Hanging-drop cultures 2. Microculture chambers 3. Soft agar (0. 75 % w/v) matrix. This is one of the better methods as it ensures support for the protoplast.

Protoplast Culture Hanging-drop cultures Microculture chambers

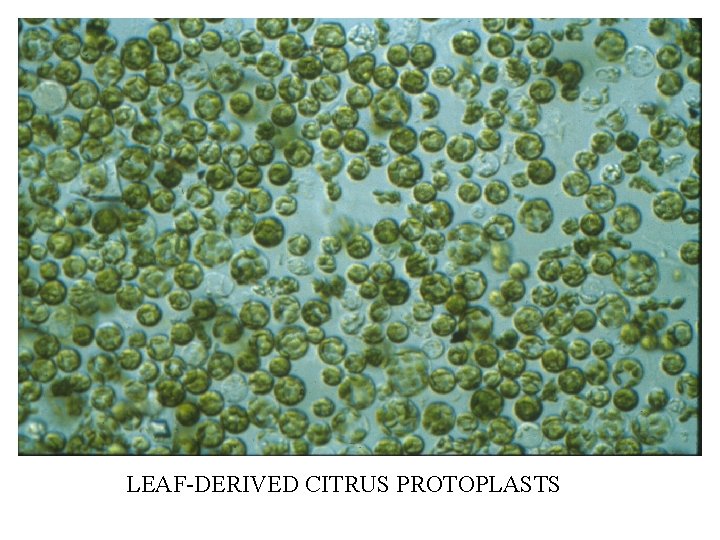
LEAF-DERIVED CITRUS PROTOPLASTS

Regeneration of Cereals I. Background - Morphogenesis is focused primarily on producing transgenic plants. Isolation, culture and maintenance of competent cells and regeneration of transgenic plants. Embryogenesis is preferred because of single cell origin. II. Phase/stages of culture leading to plant regeneration (see example) A. Induction B. Maintenance C. Regeneration D. Rooting

Regeneration of Cereals I. Background - Morphogenesis is focused primarily on producing transgenic plants. Isolation, culture and maintenance of competent cells and regeneration of transgenic plants. Embryogenesis is preferred because of single cell origin. II. Phase/stages of culture leading to plant regeneration (see example) A. Induction B. Maintenance C. Regeneration D. Rooting A. Induction - Explants are isolated that contain high frequency of competent cells and there is proliferation of pre-embryonically competent cells (PEDC), usually on medium with high auxin and, in some instances, asparagine/ proline/glutamine, examples

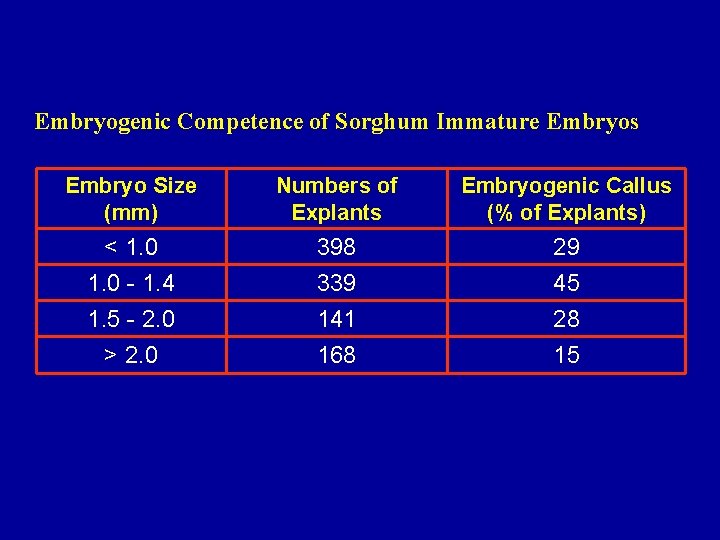
Embryogenic Competence of Sorghum Immature Embryos Embryo Size (mm) Numbers of Explants Embryogenic Callus (% of Explants) < 1. 0 - 1. 4 1. 5 - 2. 0 > 2. 0 398 339 141 168 29 45 28 15

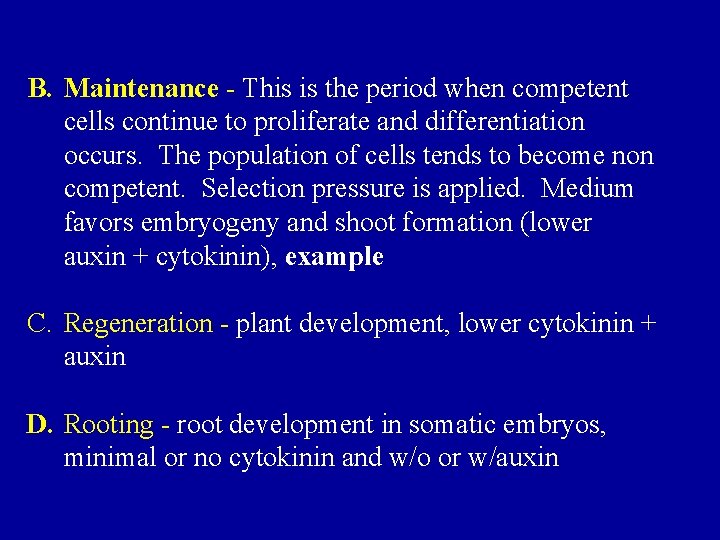
B. Maintenance - This is the period when competent cells continue to proliferate and differentiation occurs. The population of cells tends to become non competent. Selection pressure is applied. Medium favors embryogeny and shoot formation (lower auxin + cytokinin), example C. Regeneration - plant development, lower cytokinin + auxin D. Rooting - root development in somatic embryos, minimal or no cytokinin and w/o or w/auxin

Induction and Maintenance of Embryogenic Callus from Sorghum Immature Inflorescences

Regeneration of Sorghum via Somatic Embryogenesis
 Psammo culture definition
Psammo culture definition Callus culture diagram
Callus culture diagram Initiation and maintenance of callus culture
Initiation and maintenance of callus culture Significance of callus culture
Significance of callus culture Significance of callus culture
Significance of callus culture Tbc caverna
Tbc caverna Callus
Callus Callus
Callus Mr lee van rensburg
Mr lee van rensburg Fracture definiton
Fracture definiton Callus formation
Callus formation Bázis inzulin fajták
Bázis inzulin fajták Fibrocartilaginous callus formation
Fibrocartilaginous callus formation Tissue culture media types
Tissue culture media types Perforation plates
Perforation plates Plant tissue culture terminology
Plant tissue culture terminology Application of ptc
Application of ptc Conclusion for tissue culture
Conclusion for tissue culture Techniques of tissue culture
Techniques of tissue culture Explant is
Explant is Auxin in tissue culture
Auxin in tissue culture Introduction of pearl culture
Introduction of pearl culture Plant tissue culture
Plant tissue culture Primary meristem
Primary meristem Pharmacognosy scope
Pharmacognosy scope Protoplast fusion
Protoplast fusion Sclerenchyma
Sclerenchyma Three types of ground tissue
Three types of ground tissue Connective tissue function
Connective tissue function Specific tissue
Specific tissue What are the primary tissue types
What are the primary tissue types Types of tissue in the body
Types of tissue in the body Dermal tissue types
Dermal tissue types Function of elastic connective tissue
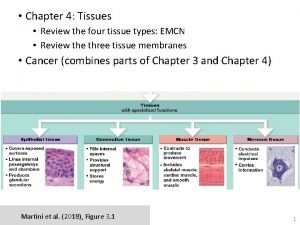
Function of elastic connective tissue Four principal types of tissue
Four principal types of tissue