Cubical atom model of Gilbert Lewis Single covalent
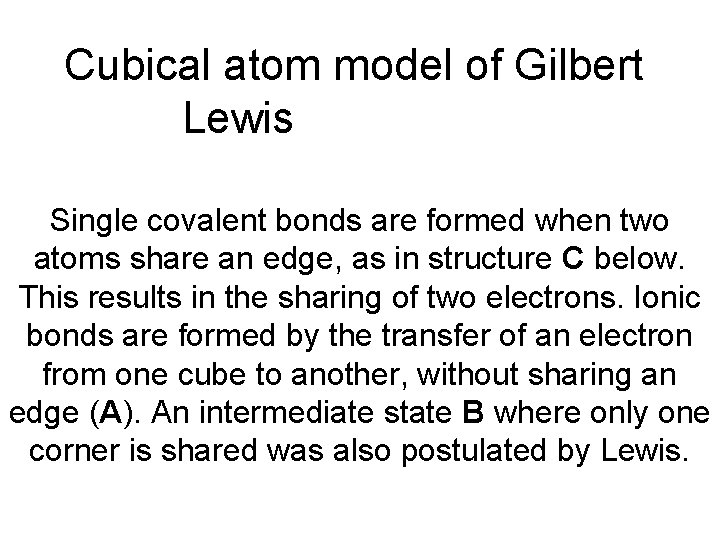
Cubical atom model of Gilbert Lewis Single covalent bonds are formed when two atoms share an edge, as in structure C below. This results in the sharing of two electrons. Ionic bonds are formed by the transfer of an electron from one cube to another, without sharing an edge (A). An intermediate state B where only one corner is shared was also postulated by Lewis.

• Double bonds are formed by sharing a face between two cubic atoms. This results in sharing four electrons:

• Triple bonds could not be accounted for by the cubical atom model, because there is no way of having two cubes share three parallel edges. Lewis suggested that the electron pairs in atomic bonds have a special attraction, which result in a tetrahedral structure, as in the figure below (the new location of the electrons is represented by the dotted circles in the middle of the thick edges). This allows the formation of a single bond by sharing a corner, a double bond by sharing an edge, and a triple bond by sharing a face. It also accounts for the free rotation around single bonds and for the tetrahedral geometry of methane. Remarkably, it could be said that there was a grain of truth in this idea, because it was later shown that the Pauli exclusion principle results in a "Fermi hole" of decreased repulsion between a pair of electrons with opposite spins in the same orbital.
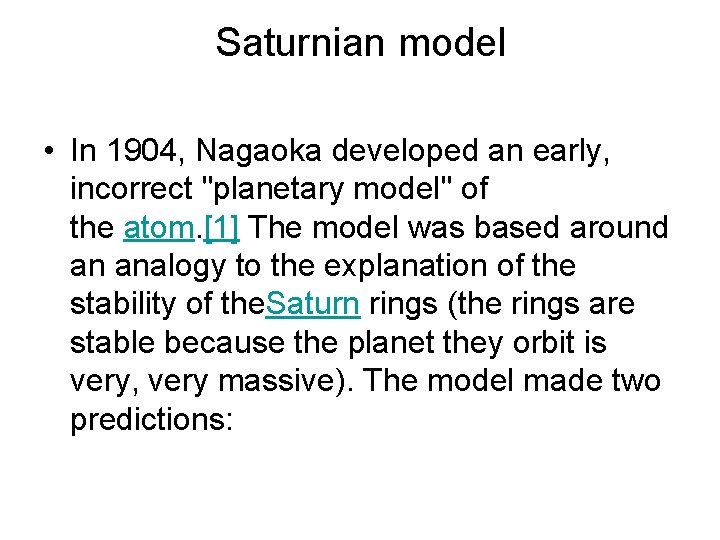
Saturnian model • In 1904, Nagaoka developed an early, incorrect "planetary model" of the atom. [1] The model was based around an analogy to the explanation of the stability of the. Saturn rings (the rings are stable because the planet they orbit is very, very massive). The model made two predictions:

• a very massive nucleus (in analogy to a very massive planet) • electrons revolving around the nucleus, bound by electrostatic forces (in analogy to the rings revolving around Saturn, bound by gravitational forces).

• Both predictions were successfully confirmed by Rutherford (who mentions Nagaoka's model in his 1911 paper in which the nucleus is proposed). However, other details of the model were incorrect (in particular, charged rings would be unstable due to repulsive disruption, which is not the case with Saturn's rings), and Nagaoka himself abandoned it in 1908.
- Slides: 6