CTSCAN MRI LIRADS 2018 Dr NGUYN H TRC









- Slides: 30

CT-SCAN & MRI LIRADS 2018 Dr. NGUYỄN HỒ TRÚC LINH - MRI

v CONTENTS: 1 2 3 4 What’s LIRADS? Which cases is LIRADS Applied and not applied for? LIRADS ’ diagnostic categories Treatment and Prognosis through LIRADS

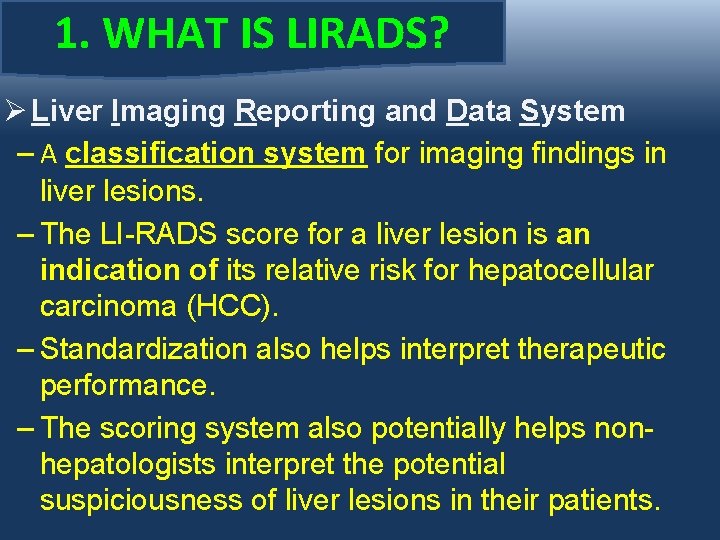
1. WHAT IS LIRADS? Ø Liver Imaging Reporting and Data System – A classification system for imaging findings in liver lesions. – The LI-RADS score for a liver lesion is an indication of its relative risk for hepatocellular carcinoma (HCC). – Standardization also helps interpret therapeutic performance. – The scoring system also potentially helps nonhepatologists interpret the potential suspiciousness of liver lesions in their patients.

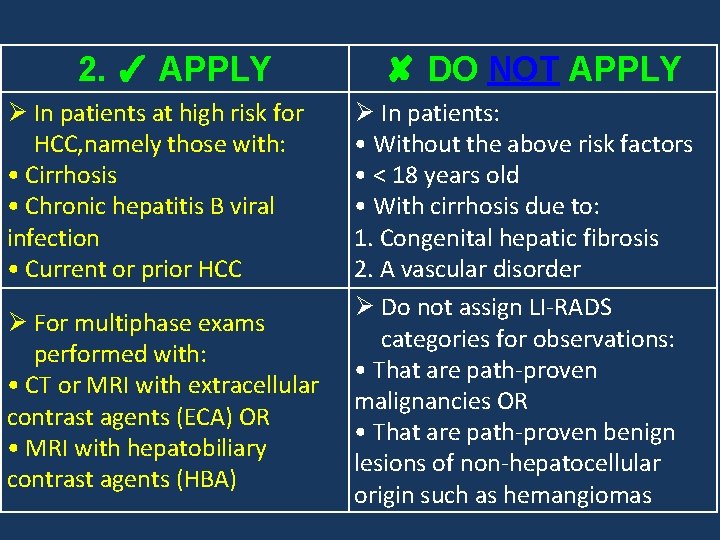
2. ✓ APPLY Ø In patients at high risk for HCC, namely those with: • Cirrhosis • Chronic hepatitis B viral infection • Current or prior HCC Ø For multiphase exams performed with: • CT or MRI with extracellular contrast agents (ECA) OR • MRI with hepatobiliary contrast agents (HBA) ✘ DO NOT APPLY Ø In patients: • Without the above risk factors • < 18 years old • With cirrhosis due to: 1. Congenital hepatic fibrosis 2. A vascular disorder Ø Do not assign LI-RADS categories for observations: • That are path-proven malignancies OR • That are path-proven benign lesions of non-hepatocellular origin such as hemangiomas


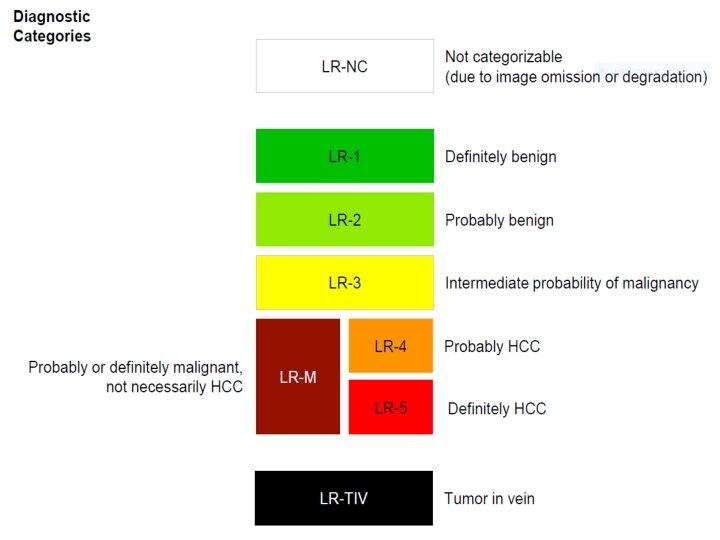
v LR-1 (100% BENIGN): Ø Imaging features diagnostic of a benign entity ü Cyst ü Hemangioma ü Vascular anomaly ü Perfusion alteration ü Hypertrophic pseudomass ü Confluent hepatic fibrosis ü Focal scar Ø Definite disappearance at follow up without treatment is also indicative of LR-1

v LR-2 (PROBABLY BENIGN): Ø Similar to LR-1. Ø A solid <20 mm nodule, without malignant / HCC / LR-M imaging features. § § T 1 Hyperintense § NO: APHE, T 2 Hypointense Washout, Siderotic Capsule, Growth HBP Hyperintense

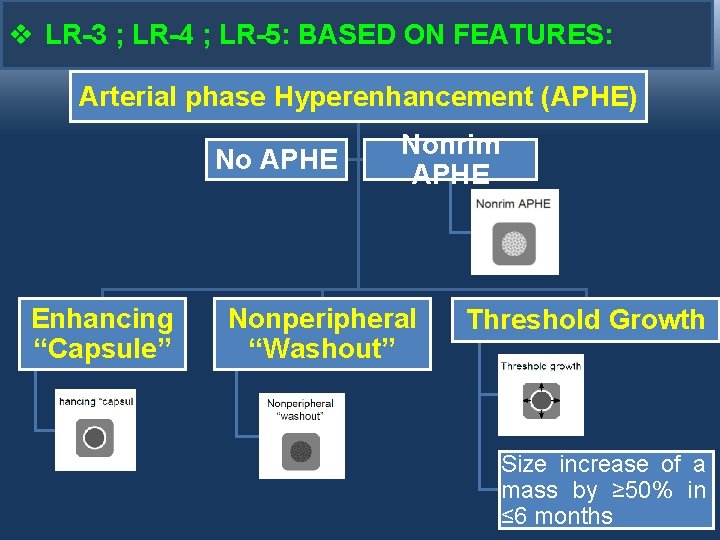
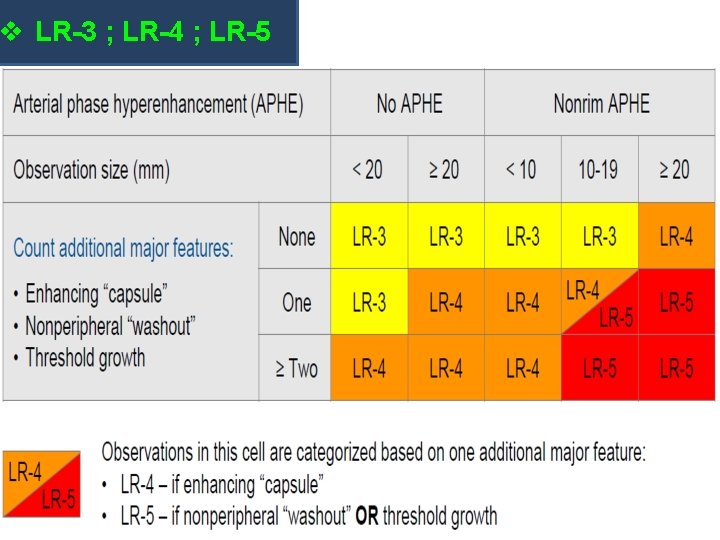
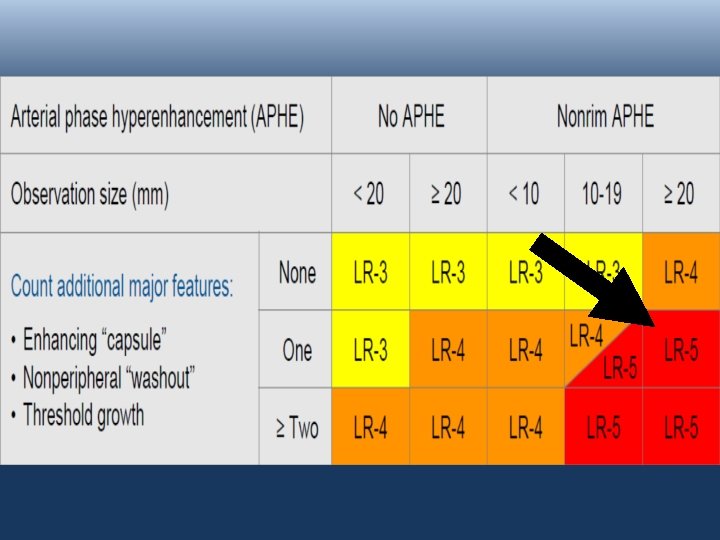
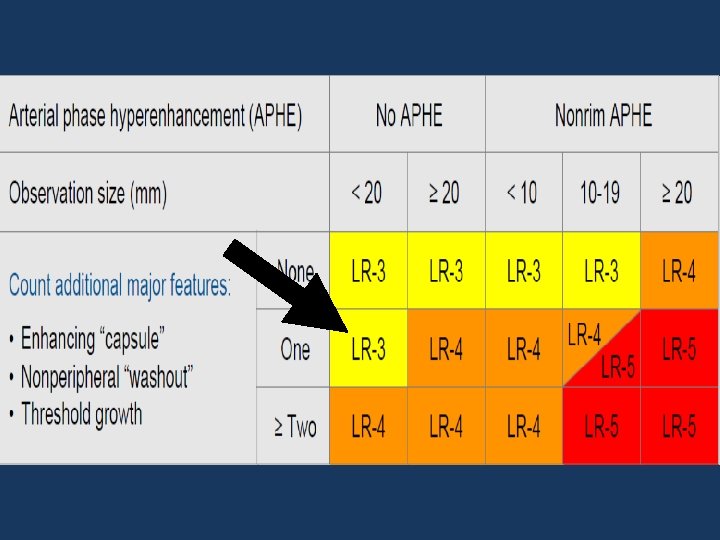
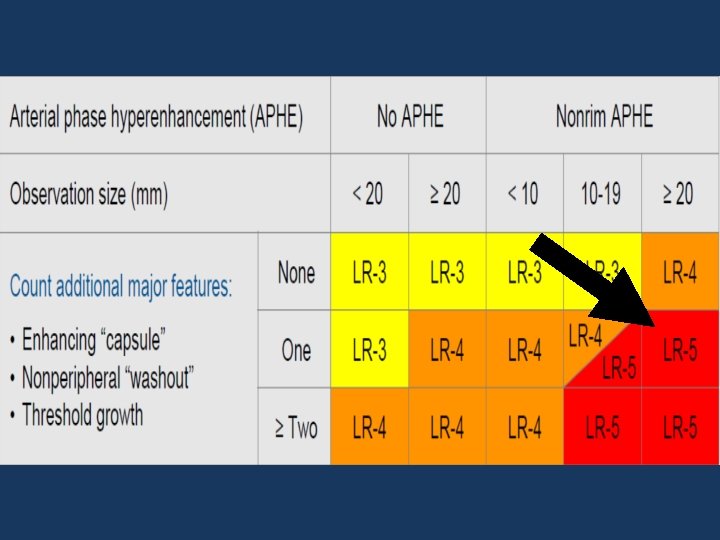
v LR-3 ; LR-4 ; LR-5: BASED ON FEATURES: Arterial phase Hyperenhancement (APHE) No APHE Enhancing “Capsule” Nonrim APHE Nonperipheral “Washout” Threshold Growth Size increase of a mass by ≥ 50% in ≤ 6 months

v LR-3 ; LR-4 ; LR-5

v SPECIAL CATEGORIES: LR-NC (LR-Non Categorizable): ØFor lesions in which the technical quality of the MRI does not allow evaluation of the major features.

v SPECIAL CATEGORIES: q LR-M: Ø For liver lesions that are probably or definitely malignant, but not an appearance compatible with HCC.

v SPECIAL CATEGORIES: q LR-TIV: Ø For unequivocal enhancing soft tissue invading the portal vein, regardless of whether an underlying parenchymal mass is visible. Ø This is important to report since it is a contraindication to liver transplantation. Ø Malignancies other than HCC can invade the portal venous system.

CASES

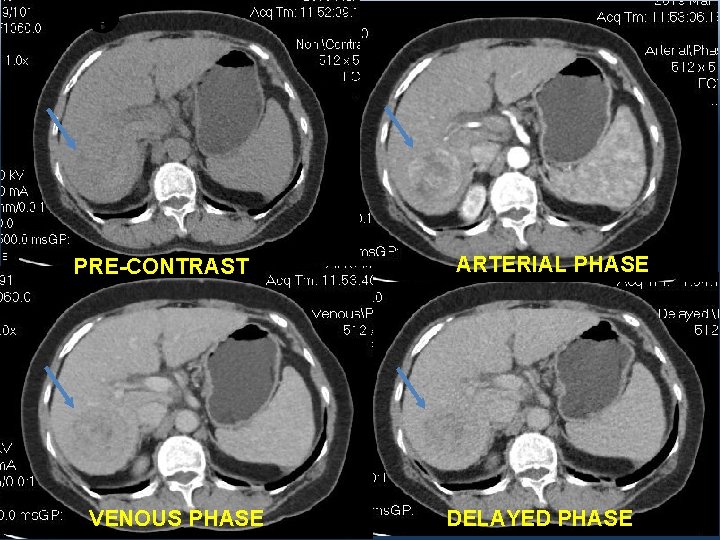
1 ST CASE: • A 65 y. o female patient, with a medical history of Cirrhosis. • AFP: 1194, 8 ng/ml; L 3: 8, 7%; DCP: 5801 m. AU/m. L. • CT-Scan: a 50 mm lesion in the right hepatic lobe.

PRE-CONTRAST VENOUS PHASE ARTERIAL PHASE DELAYED PHASE


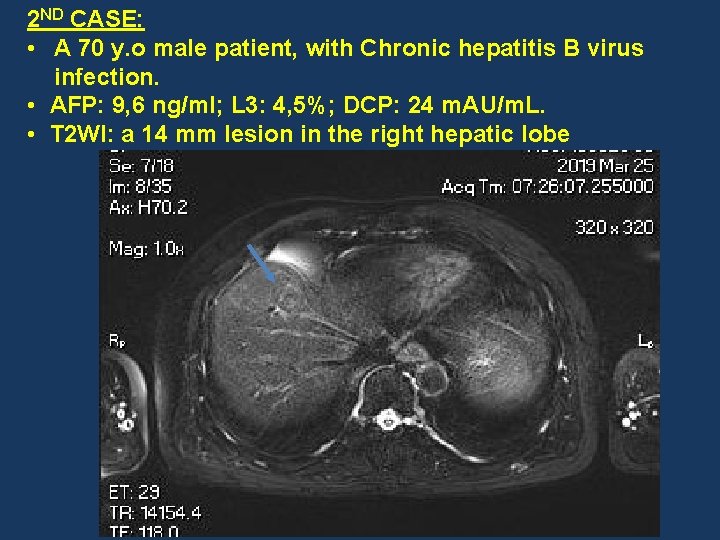
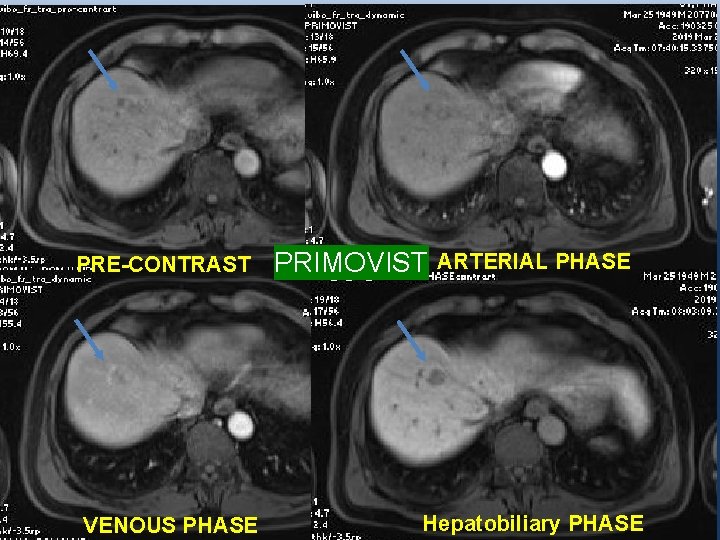
2 ND CASE: • A 70 y. o male patient, with Chronic hepatitis B virus infection. • AFP: 9, 6 ng/ml; L 3: 4, 5%; DCP: 24 m. AU/m. L. • T 2 WI: a 14 mm lesion in the right hepatic lobe

PRE-CONTRAST VENOUS PHASE PRIMOVIST ARTERIAL PHASE Hepatobiliary PHASE


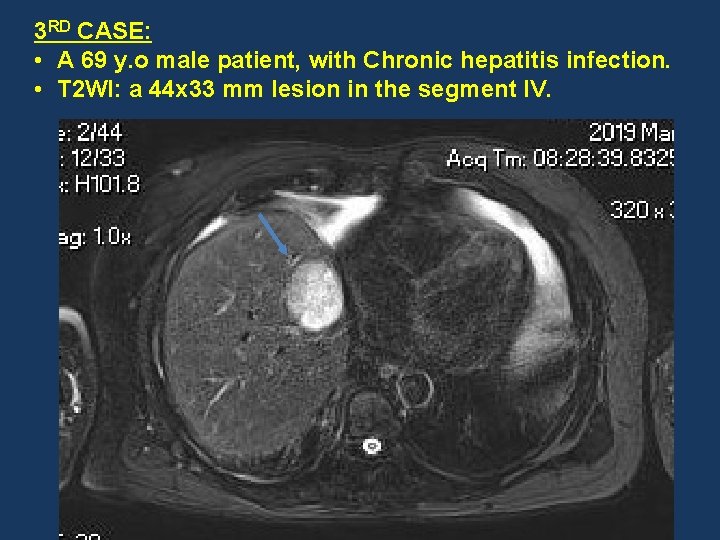
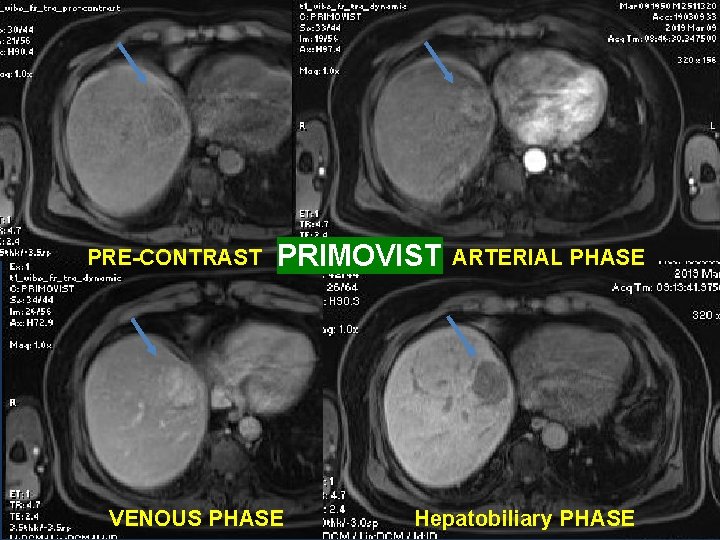
3 RD CASE: • A 69 y. o male patient, with Chronic hepatitis infection. • T 2 WI: a 44 x 33 mm lesion in the segment IV.

PRE-CONTRAST PRIMOVIST VENOUS PHASE ARTERIAL PHASE Hepatobiliary PHASE


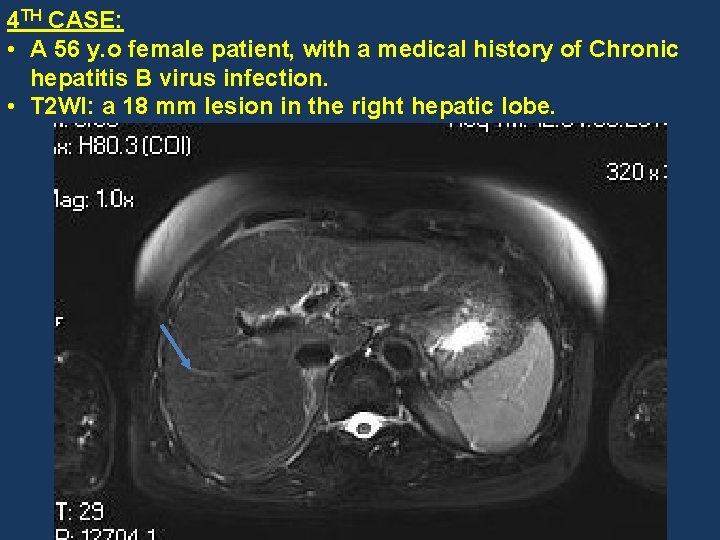
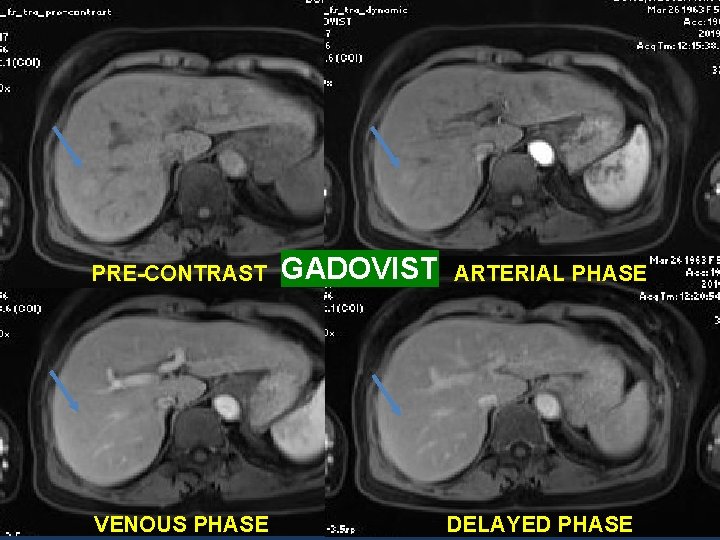
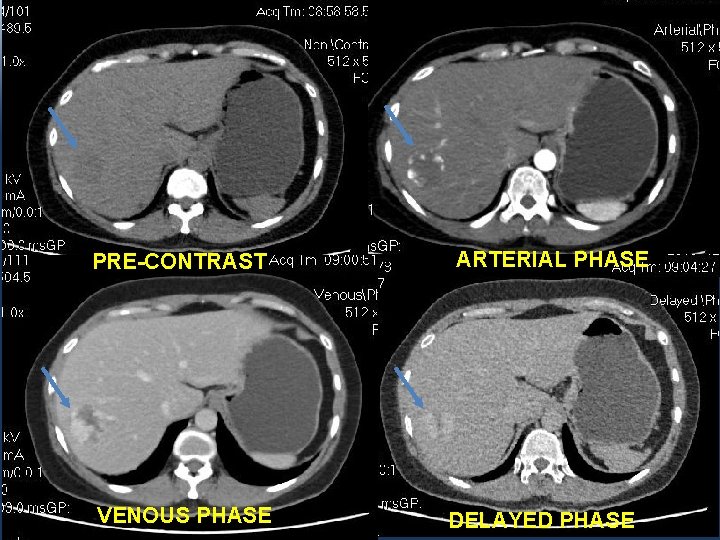
4 TH CASE: • A 56 y. o female patient, with a medical history of Chronic hepatitis B virus infection. • T 2 WI: a 18 mm lesion in the right hepatic lobe.

PRE-CONTRAST VENOUS PHASE GADOVIST ARTERIAL PHASE DELAYED PHASE

v LR-2 (PROBABLY BENIGN) Ø Similar to LR-1. Ø A solid <20 mm nodule, without malignant / HCC / LR-M imaging features. § § T 1 Hyperintense § NO: APHE, T 2 Hypointense Washout, Siderotic Capsule, Growth HBP Hyperintense

5 TH CASE: • A 49 y. o male patient, with Chronic hepatitis infection • CT-Scan: a 37 mm lesion in the right hepatic lobe.

PRE-CONTRAST VENOUS PHASE ARTERIAL PHASE DELAYED PHASE

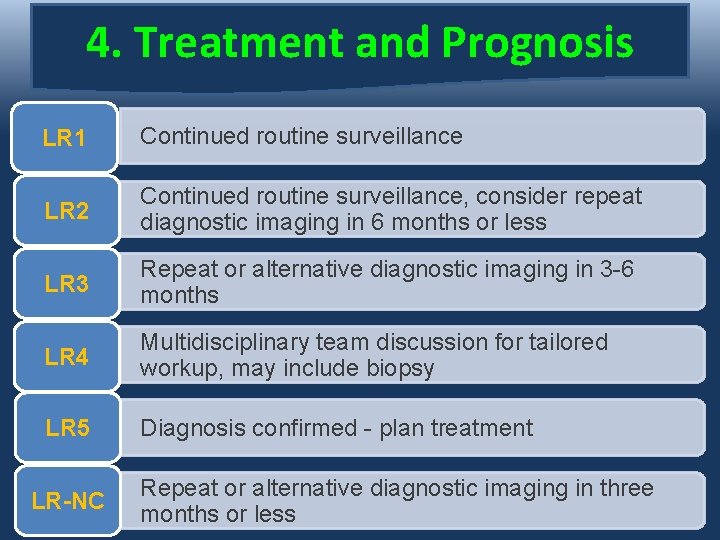
4. Treatment and Prognosis LR 1 Continued routine surveillance LR 2 Continued routine surveillance, consider repeat diagnostic imaging in 6 months or less LR 3 Repeat or alternative diagnostic imaging in 3 -6 months LR 4 Multidisciplinary team discussion for tailored workup, may include biopsy LR 5 Diagnosis confirmed - plan treatment LR-NC Repeat or alternative diagnostic imaging in three months or less

References: Ø https: //radiopaedia. org/articles/li-rads? lang=us Ø https: //www. acr. org/Clinical. Resources/Reporting-and-Data-Systems/LIRADS/CT-MRI-LI-RADS-v 2018

THANKS FOR LISTENING!