CS Phase III Study in Nigeria Funder USAID







- Slides: 10

CS Phase III Study in Nigeria Funder: USAID Sponsor: CONRAD Research Organization: FHI Vera Halpern, MD Principal Investigator Collaborators in Nigeria: Folasade Ogunsola, MD College of Medicine, University of Lagos OK Obunge, MD Teaching Hospital, University of Port Harcourt

Product Under Investigation § 6% Sodium Cellulose Sulfate (CS) ü ü ü high molecular weight polymer inhibitor of entry/fusion potential contraceptive § Vaginal gel § Single-use applicator § 3. 5 ml of gel

Study Objectives Primary: n Determine the effectiveness of CS gel in preventing vaginal transmission of HIV Secondary: n Determine the effectiveness of CS gel in preventing vaginal transmission of gonorrhea and chlamydia

Study Design n Phase 3 randomized placebo-controlled trial n n n 2160 women at high risk of HIV/STIs n n 1: 1 allocation; condoms in both arms no condom only arm 1080 women in Lagos 1080 women in Port Harcourt Follow up: 12 months Test for HIV, gonorrhea and chlamydia at baseline and at each monthly follow up visit

CS/Nigeria Study Timeline Highlights n December 2004 – initiation of screening n October 2006: n n 70% of enrollment completed in Nigeria Enrollment is stopped in Nigeria Plans to expand trial to South Africa December 2008 – estimated end of the study

CS/Nigeria Study Timeline Highlights n January 2007: n CS/Multicountry trial is stopped by CONRAD due to apparent increased risk of HIV in CS arm at interim analysis for the DSMB n No apparent increased risk in CS/Nigeria study at interim analysis n CS/Nigeria is stopped by FHI due to safety concerns raised in CS/Multicountry study

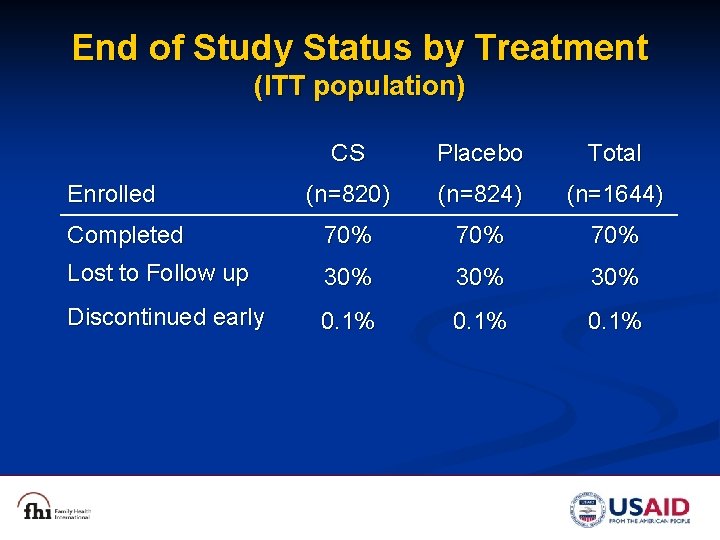
End of Study Status by Treatment (ITT population) CS Placebo Total (n=820) (n=824) (n=1644) Completed 70% 70% Lost to Follow up 30% 30% Discontinued early 0. 1% Enrolled

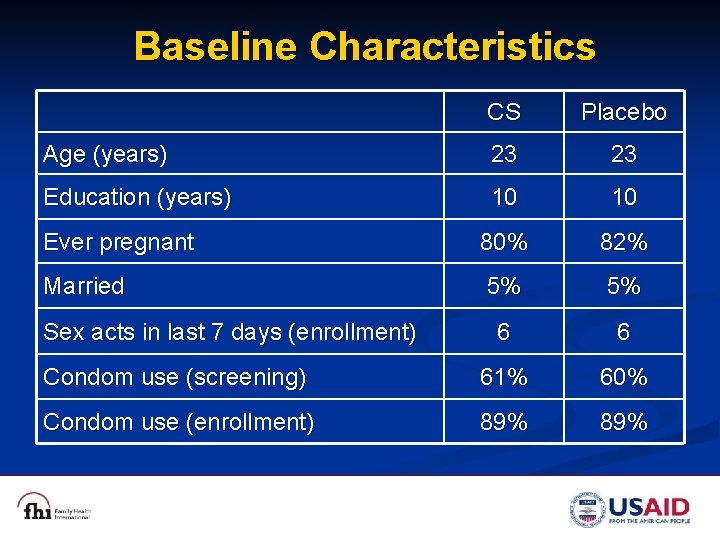
Baseline Characteristics CS Placebo Age (years) 23 23 Education (years) 10 10 Ever pregnant 80% 82% Married 5% 5% 6 6 Condom use (screening) 61% 60% Condom use (enrollment) 89% Sex acts in last 7 days (enrollment)

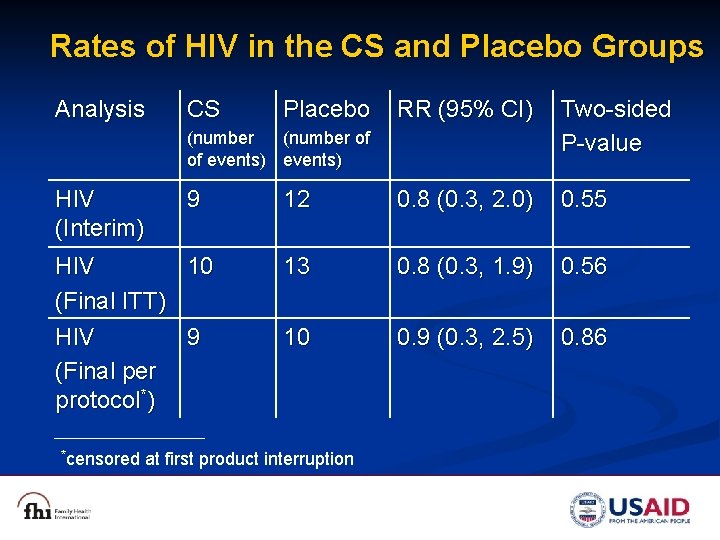
Rates of HIV in the CS and Placebo Groups Analysis CS Placebo RR (95% CI) Two-sided P-value 12 0. 8 (0. 3, 2. 0) 0. 55 13 0. 8 (0. 3, 1. 9) 0. 56 10 0. 9 (0. 3, 2. 5) 0. 86 (number of of events) HIV (Interim) 9 HIV 10 (Final ITT) HIV 9 (Final per protocol*) *censored at first product interruption

Conclusion n We did not observe an effect of CS gel on the risk of vaginal HIV transmission