Crystals Crystals A crystal is a solid in






















- Slides: 23

Crystals

Crystals A crystal is a solid in which the atoms are arranged in orderly, repeating patterns. Crystalline structure can be seen either on the inside or the outside of a mineral. Minerals that form with large amounts of space are able to arrange themselves in crystal form on the outside. If a mineral forms with limited space, the crystal structure is seen on the inside. We cannot see this with the naked eye.


Crystals from Magma is hot melted rock. (when it reaches the earth’s surface it’s called lava) When The magma cools it forms crystals rate at which the magma cools determines the size of the crystals

These are all pictures of granite. Why do they all look different?

Crystal Size The elements present in the magma will determine which minerals form The slower the magma cools, the larger the individual crystals The quicker the magma cools, the smaller the individual crystals

Crystals from Solution Another way we can get crystals is from a solution Remember, a solution is a mixture of two or more things that are not chemically combined Sea water is a solution When the water evaporated, crystals are left behind.

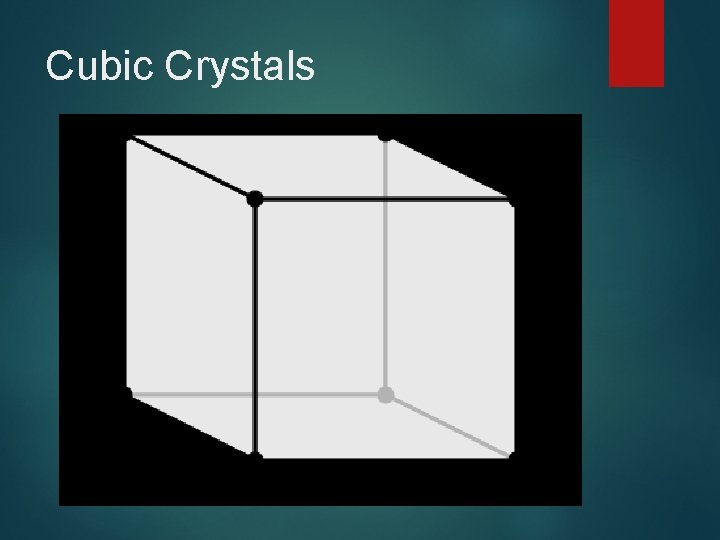
Cubic Crystals

Cubic Crystals Cubic crystals have all 90 degree angles with all sides of equal length

Examples of cubic crystals Galena

Cubic Crystals Halite

Halite

6 -sided crystal Sturmanite Hexagonal Crystal

Hexagonal Crystals Quartz

Tetragonal Crystals Much like the cubic, except one side is longer than the others, like a rectangle. Zircon is a good example

Think of this as a brick. It’s like the last one, a rectangle that has been flattened so that its thicknesses are different. Orthorhombic

Orthorhombic has all 90° angles still. The rectangle is flattened either a little or a lot, as seen here in a sample of barite. All 3 (pairs of) sides have different lengths.

Monoclinic Take the ‘brick, ’ orthorhombic, and slide it askew so that only one angle is left at a 90° angle Orthoclase is an example of a monoclinic crystal shape

Monoclinic crystal: Orthoclase

Triclinic This No is an unsymmetrical as a mineral can get 90°angles Rhodonite is an example

More rhodonite

Rhodonite

Overview All minerals have crystalline structure; either on the inside or outside With plenty of room they form on the outside If confined the crystals are in the atomic structure Crystal size is dependent on how quickly (small crystals) or slowly (large crystals) they form Crystals can form from magma or from solution There are 6 basic crystal shapes 5 of these are versions of cubes, the 6 th is a hexagon All minerals are composed of crystals. Minerals are “naturally occurring, inorganic solids with definite chemical composition and orderly arrangement of atoms. ”