CRYSTALLIZATION Definition CrystallizationSpontaneous arrangement of the particles into







- Slides: 23

CRYSTALLIZATION

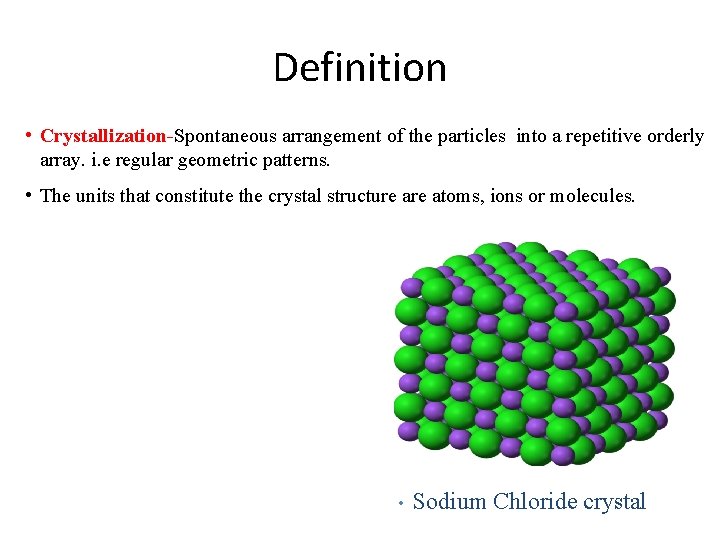
Definition • Crystallization-Spontaneous arrangement of the particles into a repetitive orderly array. i. e regular geometric patterns. • The units that constitute the crystal structure atoms, ions or molecules. • Sodium Chloride crystal

Ø Objectives and applications Purification of Drugs – removing of impurities. Ø Better processing characteristics- improved micromeritics such as compressibility and wettability. Ø Ease of handling-facilitation of transportation and storage. Ø Better Chemical stability-Amorphous Penicillin G is less stable than crystalline salt. Ø Improved Physical stability-such as suspension stability and hardness of tablet. Ø Improved bioavailability- Crystalline Penicillin G does not dissolve immediately in gastric fluids, therefore its degradation decreases and bioavailability increases. Ø Sustained release-Protamine zinc insulin in crystalline form slowly and continously releases insulin from site of injection.

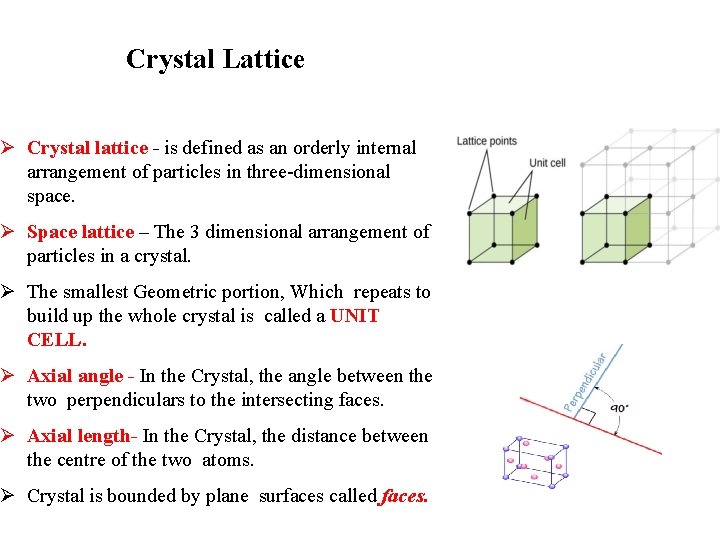
Crystal Lattice Ø Crystal lattice - is defined as an orderly internal arrangement of particles in three-dimensional space. Ø Space lattice – The 3 dimensional arrangement of particles in a crystal. Ø The smallest Geometric portion, Which repeats to build up the whole crystal is called a UNIT CELL. Ø Axial angle - In the Crystal, the angle between the two perpendiculars to the intersecting faces. Ø Axial length- In the Crystal, the distance between the centre of the two atoms. Ø Crystal is bounded by plane surfaces called faces.

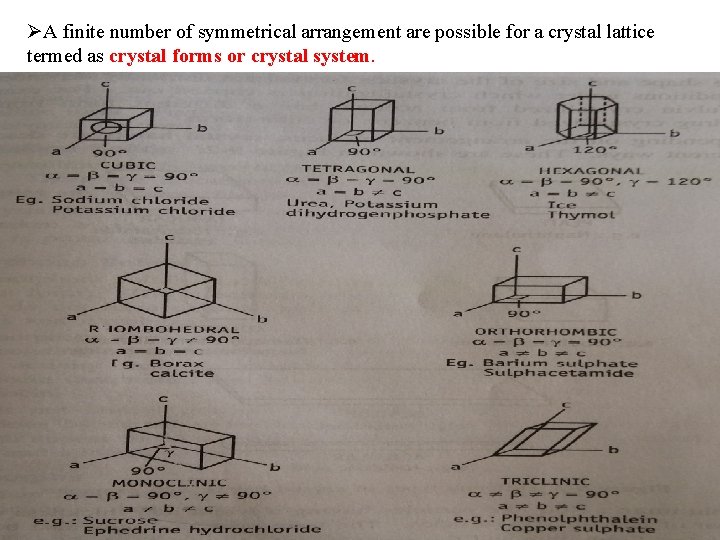
ØA finite number of symmetrical arrangement are possible for a crystal lattice termed as crystal forms or crystal system.

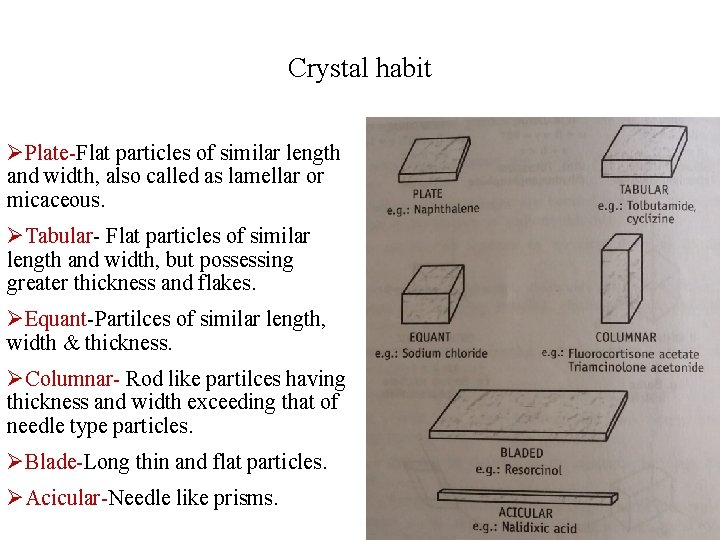
Crystal habit ØPlate-Flat particles of similar length and width, also called as lamellar or micaceous. ØTabular- Flat particles of similar length and width, but possessing greater thickness and flakes. ØEquant-Partilces of similar length, width & thickness. ØColumnar- Rod like partilces having thickness and width exceeding that of needle type particles. ØBlade-Long thin and flat particles. ØAcicular-Needle like prisms.

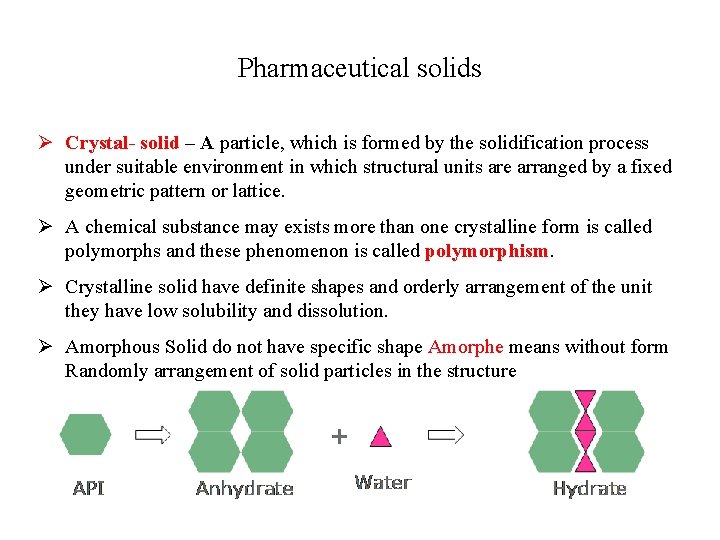
Pharmaceutical solids Ø Crystal- solid – A particle, which is formed by the solidification process under suitable environment in which structural units are arranged by a fixed geometric pattern or lattice. Ø A chemical substance may exists more than one crystalline form is called polymorphs and these phenomenon is called polymorphism. Ø Crystalline solid have definite shapes and orderly arrangement of the unit they have low solubility and dissolution. Ø Amorphous Solid do not have specific shape Amorphe means without form Randomly arrangement of solid particles in the structure Ø CRYSTAL HYDRATES Some drugs have greater tendency to associate with water called Drug hydrates e. g. Na 2 CO 3. 10 H 2 O

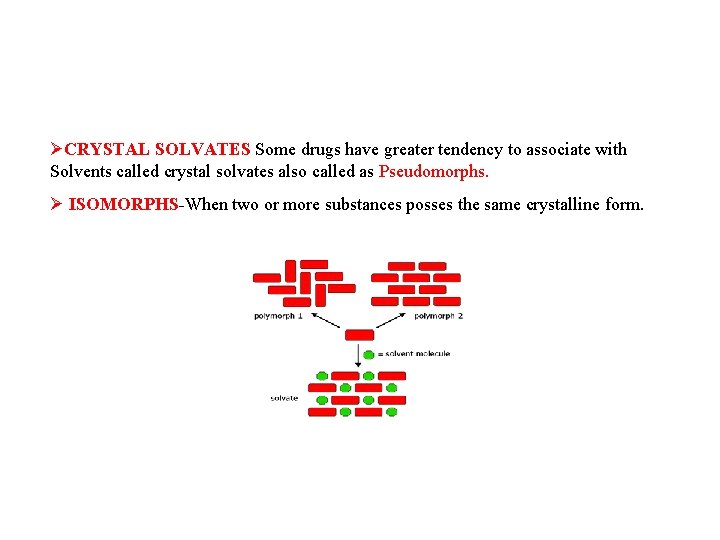
ØCRYSTAL SOLVATES Some drugs have greater tendency to associate with Solvents called crystal solvates also called as Pseudomorphs. Ø ISOMORPHS-When two or more substances posses the same crystalline form.

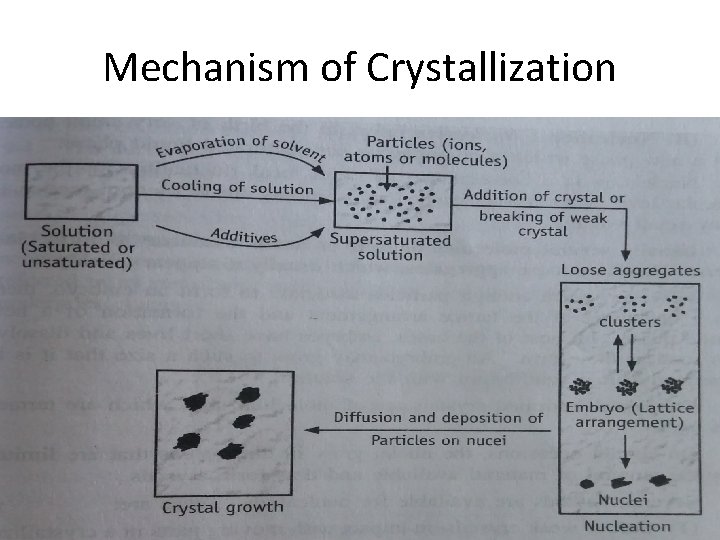
Mechanism of Crystallization

Mechanism of Crystallization Ø A. Supersaturation When the concentration of a compound in its solution is greater than the saturation solubility of that compound in that solvent the condition Ø B. Nucleation-birth of very small bodies of molecules from which the crystal forms. When the solute particles (molecules, atoms or ions) moves and collide over each other they may form aggregates. Ø This aggregates are called clusters. These are loose aggregates, which usually disappear quickly. Ø Some clusters may become so big that they may arrange themselves in lattice arrangement. These bodies of aggregates are called embryo. Ø Some embryo may grow to a bigger size are called as nucleus (plural is nuclei). Ø C. Crystal growth is a diffusion process and a surface phenomenon. Every crystal is surrounded by a layer of liquid known as stagnant layer. From the bulk solution a solute particle (molecule, atom or ion) diffuse through this stagnant layer and then reaches the surface of the crystal.

Factors affecting the crystal habit Ø Presence of another substance in the mother liquor: Sodium chloride crystallized from aqueous solutions produces cubic crystals. If sodium chloride is crystallized from a solution containing a small amount of urea, the crystals obtained will have octahedral faces. Both types of crystals belong to the cubic crystal form but differ in habit. Ø Solvent: Griseofulvin crystallized out from acetone has different crystal habit than when crystallized from benzene or chloroform. Ø Rate of cooling: Acicular or needle-like crystals are produced when the solution is cooled very slowly. Fluffy and small crystals are produced when the solution is cooled very fast. Ø Slow Evaporation/Slow Cooling: Ø Time – faster crystallization is not as good as slow crystallization. Ø Faster crystallization higher chance of lower quality crystals Ø o Quality crystals grow best over time in near equilibrium conditions Ø o The longer the time, the better the crystals.

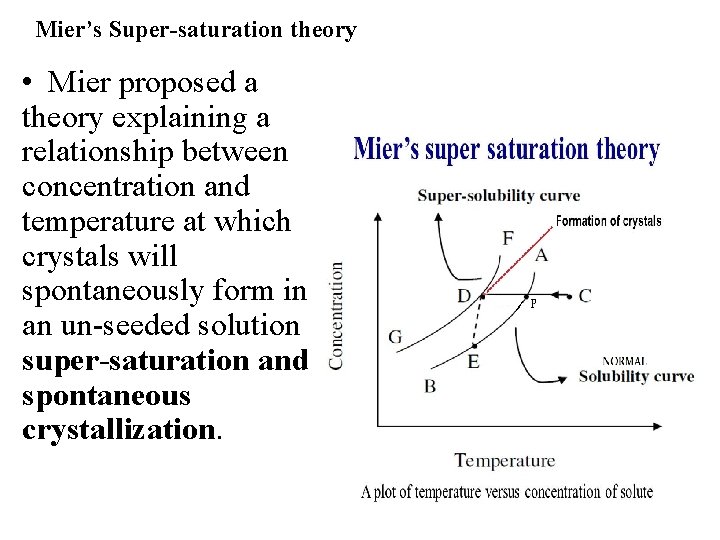
Mier’s Super-saturation theory • Mier proposed a theory explaining a relationship between concentration and temperature at which crystals will spontaneously form in an un-seeded solution super-saturation and spontaneous crystallization.

Mier’s Super-saturation theory • The theory can be explained with the help of solubilty - supersolubility diagram. • Here the curve AB is the normal solubility (equilibrium) curve. Any point on curve represents the solute in equilibrium with solvent. This is the max limit for solubility of a substance. • The curve FG represents the super solubility which is roughly parallel to the normal solubility curve. • This is the point at which nucleus formation begins spontaneously. • The region between AB and FG is metastable state. i. e the system is unstable and undergoes changes. • According to mier’s theory crystallization do not start at P but it takes place somewhere in the neighborhood of point D.

CLASSIFICATION OF CRYSTALLIZERS • • • Based on the method employed for producing the supersaturated solution. AGITATED BATCH CRYSTALLIZER SWENSON WALKER CRYSTALLIZER KRYSTAL CRYSTALLIZER VACCUM CRYSTALLIZER

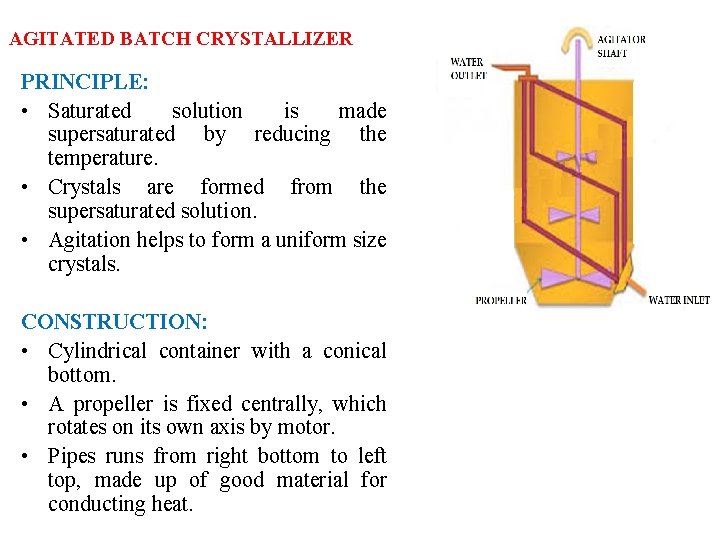
AGITATED BATCH CRYSTALLIZER PRINCIPLE: • Saturated solution is made supersaturated by reducing the temperature. • Crystals are formed from the supersaturated solution. • Agitation helps to form a uniform size crystals. CONSTRUCTION: • Cylindrical container with a conical bottom. • A propeller is fixed centrally, which rotates on its own axis by motor. • Pipes runs from right bottom to left top, made up of good material for conducting heat.

AGITATED BATCH CRYSTALLIZER WORKING: Solution is placed in the crystallizer. Cold water is passed through the pipes continuously due to this cooling, solution becomes supersaturated and crystals are formed. Propeller is allowed to rotate. It has two purposes 1. It increases rate of heat transfer – maintains the temp. of the soln. uniformly. 2. It keeps fine crystals in suspension – uniform crystals. The crystals are formed at the bottom, separated from mother liquor by a suitable mechanism. ADVANTAGES : Uniform and fine crystals are formed. DISADVANTAGES : Batch or discontinuous equipment Solubility is least at the surface of the cooling coils due to decrease in the rate of heat transfer.

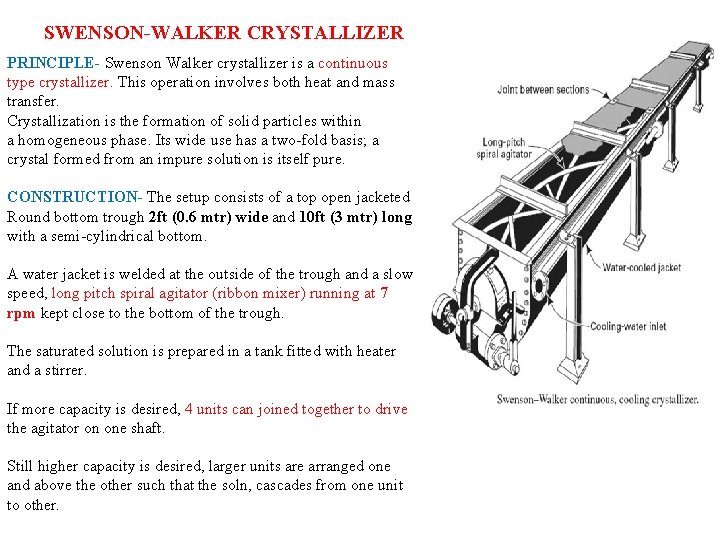
SWENSON-WALKER CRYSTALLIZER PRINCIPLE- Swenson Walker crystallizer is a continuous type crystallizer. This operation involves both heat and mass transfer. Crystallization is the formation of solid particles within a homogeneous phase. Its wide use has a two-fold basis; a crystal formed from an impure solution is itself pure. CONSTRUCTION- The setup consists of a top open jacketed Round bottom trough 2 ft (0. 6 mtr) wide and 10 ft (3 mtr) long with a semi-cylindrical bottom. A water jacket is welded at the outside of the trough and a slow speed, long pitch spiral agitator (ribbon mixer) running at 7 rpm kept close to the bottom of the trough. The saturated solution is prepared in a tank fitted with heater and a stirrer. If more capacity is desired, 4 units can joined together to drive the agitator on one shaft. Still higher capacity is desired, larger units are arranged one and above the other such that the soln, cascades from one unit to other.

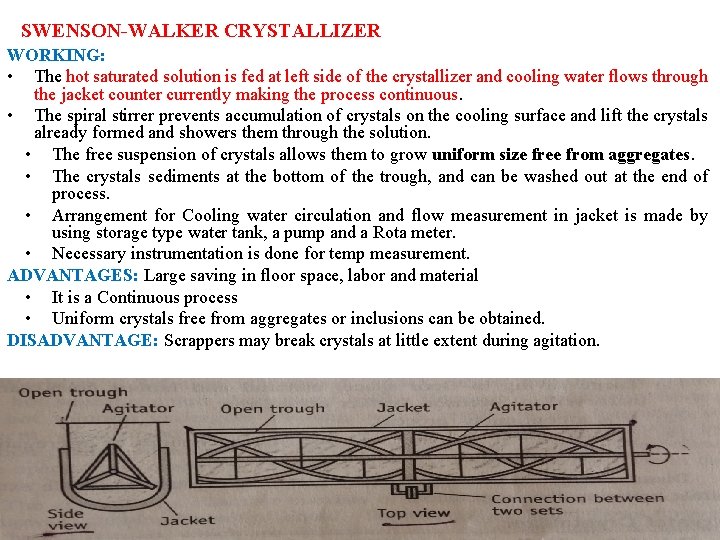
SWENSON-WALKER CRYSTALLIZER WORKING: • The hot saturated solution is fed at left side of the crystallizer and cooling water flows through the jacket counter currently making the process continuous. • The spiral stirrer prevents accumulation of crystals on the cooling surface and lift the crystals already formed and showers them through the solution. • The free suspension of crystals allows them to grow uniform size free from aggregates. • The crystals sediments at the bottom of the trough, and can be washed out at the end of process. • Arrangement for Cooling water circulation and flow measurement in jacket is made by using storage type water tank, a pump and a Rota meter. • Necessary instrumentation is done for temp measurement. ADVANTAGES: Large saving in floor space, labor and material • It is a Continuous process • Uniform crystals free from aggregates or inclusions can be obtained. DISADVANTAGE: Scrappers may break crystals at little extent during agitation.

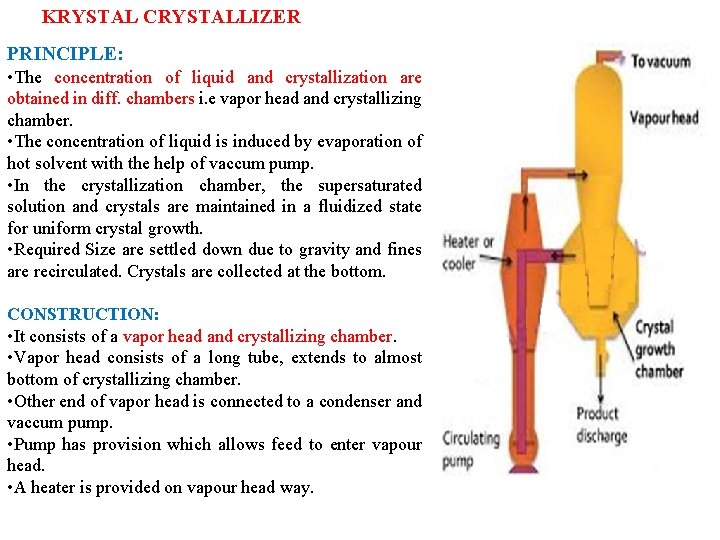
KRYSTAL CRYSTALLIZER PRINCIPLE: • The concentration of liquid and crystallization are obtained in diff. chambers i. e vapor head and crystallizing chamber. • The concentration of liquid is induced by evaporation of hot solvent with the help of vaccum pump. • In the crystallization chamber, the supersaturated solution and crystals are maintained in a fluidized state for uniform crystal growth. • Required Size are settled down due to gravity and fines are recirculated. Crystals are collected at the bottom. CONSTRUCTION: • It consists of a vapor head and crystallizing chamber. • Vapor head consists of a long tube, extends to almost bottom of crystallizing chamber. • Other end of vapor head is connected to a condenser and vaccum pump. • Pump has provision which allows feed to enter vapour head. • A heater is provided on vapour head way.

WORKING: • The solution is heated by heater and pumped into vapour head. • Hot solution undergoes flashing due to reduced pressure, which results in the formation of solvent vapour and supersaturated solution. • Vapour is removed by suction pump. • Supersaturated solution passes through the long tube. • The operation is controlled in such a way that crystals do not form in the vapour head but should form in the crystallizing chamber. • At bottom, coarse crystals are settled, fine crystals are settled above it. • Very fine crystals are overflow through the liquid and enter in to recirculating system, which then combined with fresh feed. • From time to time, coarse crystals are taken out through the opening at the bottom of the chamber. USES: Na. Cl, Mg. SO 4 crystals ADVANTAGES: Large quantities of controlled size crystals. Available in very large sizes if body- 4. 5 mtrs diameter, Ht- 6. 0 mtrs.

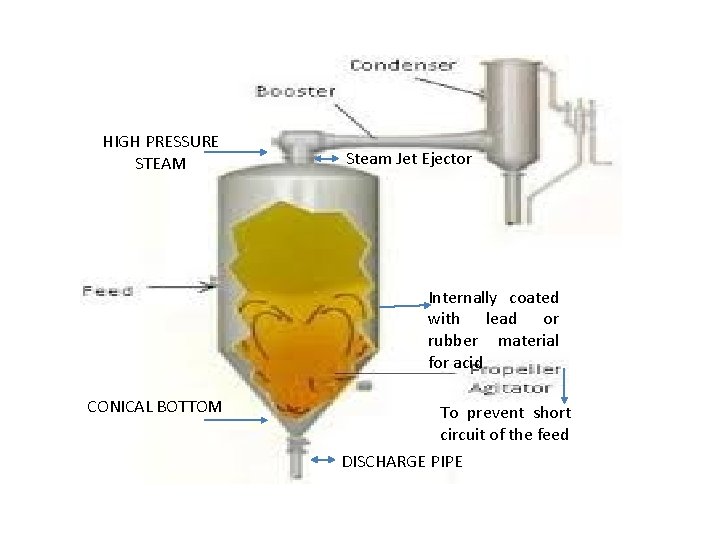
VACCUM CRYSTALLIZER PRINCIPLE: • Supersaturation is obtained by adiabetic evaporating cooling. Warm saturated solutio is introduced into the crystallizer, due to high vaccum the soln. undergoes flashing. • A part of the solvent gets evaporated. So, cooling takesplace. Resulting supersaturation, crystals are formed.

HIGH PRESSURE STEAM Steam Jet Ejector Internally coated with lead or rubber material for acid CONICAL BOTTOM To prevent short circuit of the feed DISCHARGE PIPE

WORKING: • High Vaccum is created, it must correspond to B. P. of the soln, but lower than the feed temp. • Soln. undergoes flashing, results into solvent evaporation. • Flashing leads to ebullition i. e keeps in suspended until they become large size. • Propeller prevents the soln reaching at discharge pump. • Adiabatic condns makes the body cool. This, cooling causes supersaturation and crystallization. USES: Thermolabile substances ADVANTAGES: • Very simple – no moving parts • Corrosive materials can be used • Constructed as large size • Operated either batch or large scale.