Crystal Structure of a Polyethylene OxideUrea Inclusion Compound










- Slides: 22

Crystal Structure of a Polyethylene Oxide-Urea Inclusion Compound: the End of a 45 -year-old Story Laura Adduci Professor Bruce Foxman Brandeis University April 23, 2009

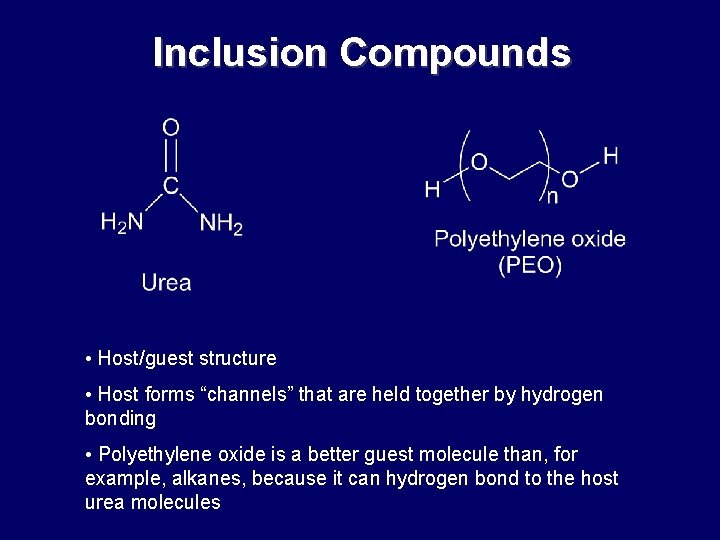
Inclusion Compounds • Host/guest structure • Host forms “channels” that are held together by hydrogen bonding • Polyethylene oxide is a better guest molecule than, for example, alkanes, because it can hydrogen bond to the host urea molecules

Background: 1964 Reported that a Polyethylene-oxide inclusion compound had been synthesized No crystal structure Reported hexagonal crystals Suggested 9: 4 ratio of PEO to urea molecules

Background: the 1991 paper

Background: the Li Di thesis

Acquisition of New Reflection Data • Saturated solutions of Polyethylene Oxide (average molecular weight 3400 g/mol) and Urea in ethanol were mixed in equal amounts • • Stirred for 2 hours Allowed to evaporate at room temperature over the course of 3 days Suitable crystal was selected and cut to an appropriate size X-ray diffraction data was gathered at 120 K on a Bruker-Nonius Kappa Apex II diffractometer equipped with Mo radiation • Collected ______ reflections, as opposed to the ___ reflections from the 1991 paper and ____ reflections for the Li Di thesis

Current Work 1 Started with Li Di’s thesis (structure A) and the 1991 Macromolecules paper (structure B). Transformed the coordinates of the Li Di structure so that they matched those in the 1991 structure. Refined both structures separately. Decided to refine the nonhydrogen urea atoms anisotropically and all other atoms isotropically.

Current Work 2 Imported my new reflections into each solution. Refined. Problem: CRYSTALS automatically detects atoms that are close to special positions and puts them on the special positions. Solution: Manually specify which should be on special positions. Attempt to restrain bond lengths and angles – unsuccessful. Combine.

Current Work 3 More problems. Return to modified Structure A. Try to figure out connectivity of the polymer chain: easy for Structure B because end atoms are on two-fold rotation axes. For Structure A: must examine bond lengths and angles to choose the most logical atom.

Current Work 4 Started with Li Di’s thesis and the 1991 Macromolecules paper. Refined both structures separately.

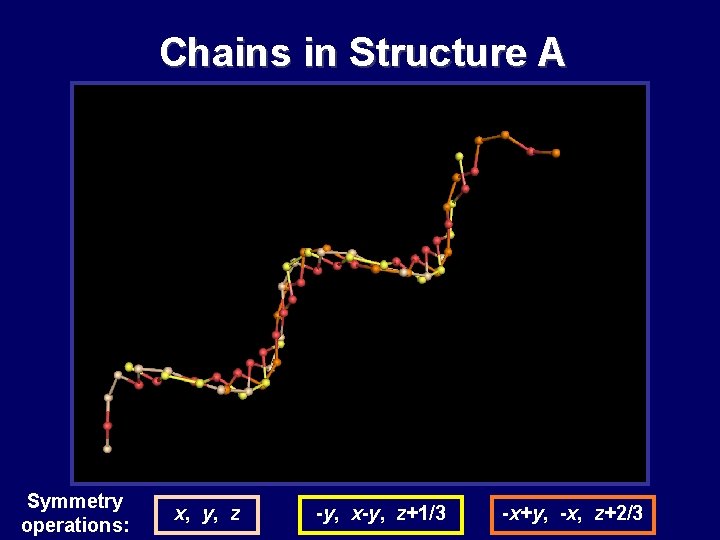
Chains in Structure A Symmetry operations: x, y, z -y, x-y, z+1/3 -x+y, -x, z+2/3

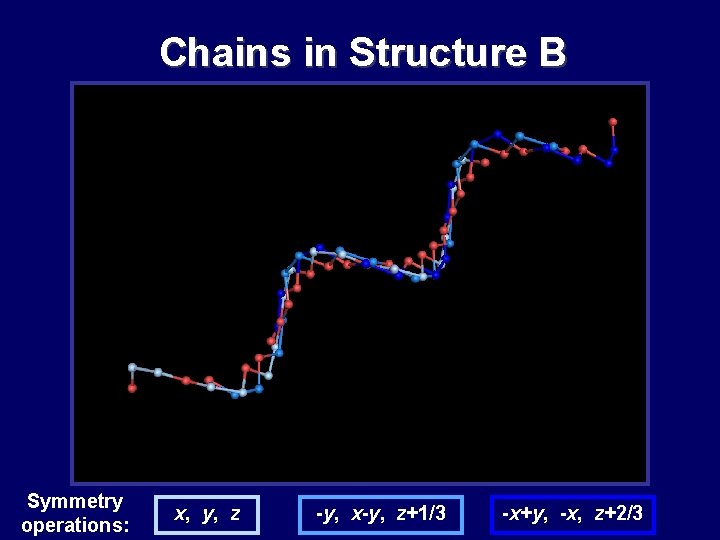
Chains in Structure B Symmetry operations: x, y, z -y, x-y, z+1/3 -x+y, -x, z+2/3

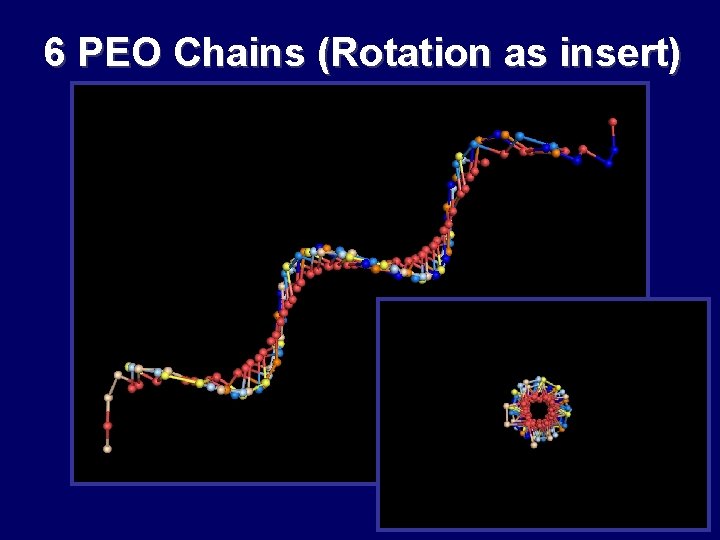
6 PEO Chains (Rotation as insert) Structure BB and A Structures

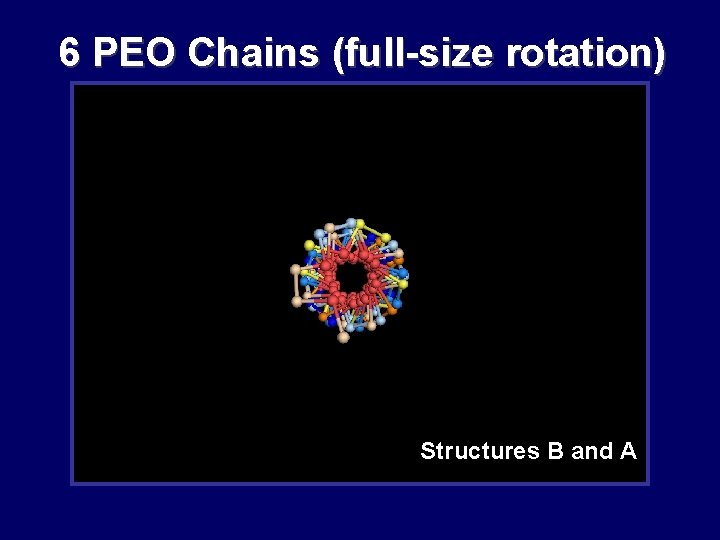
6 PEO Chains (full-size rotation) Structures B and Structure B A

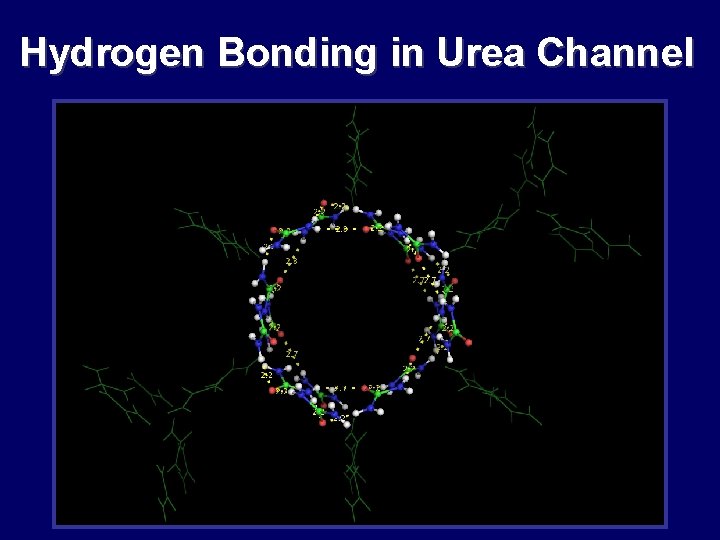
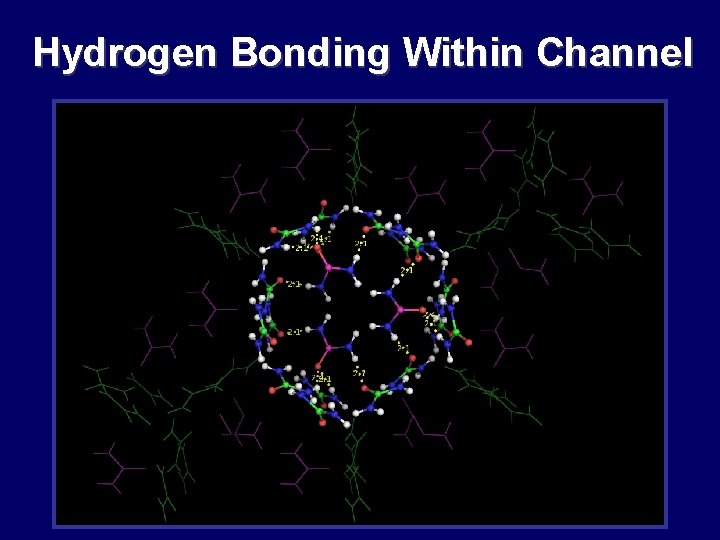
Hydrogen Bonding in Urea Channel

Hydrogen Bonding Within Channel

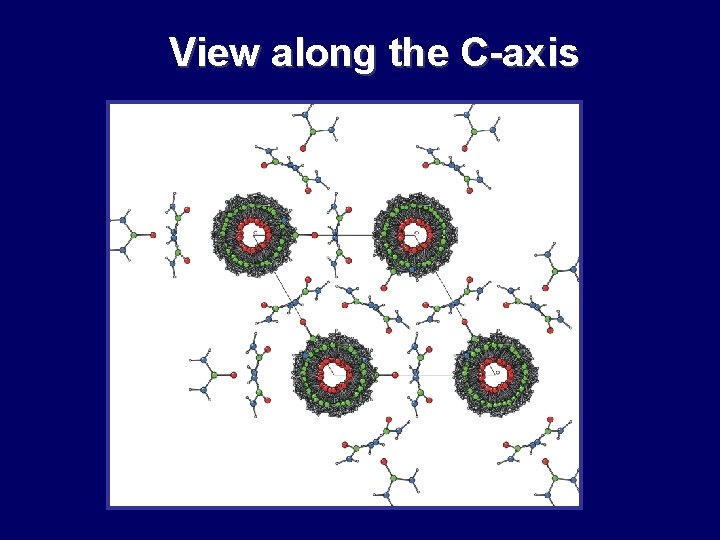
View along the C-axis

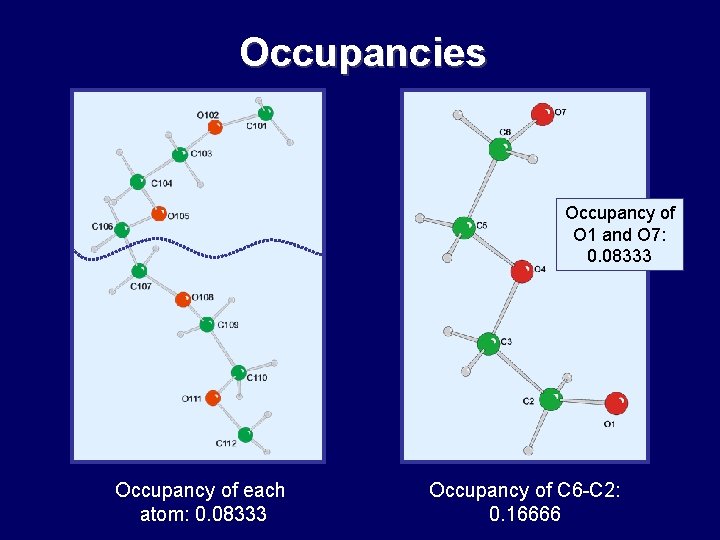
Occupancies Occupancy of O 1 and O 7: 0. 08333 Occupancy of each atom: 0. 08333 Occupancy of C 6 -C 2: 0. 16666

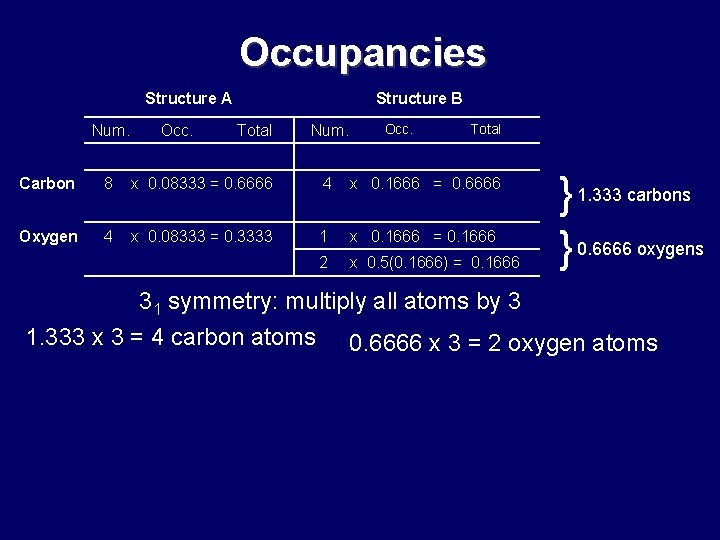
Occupancies Structure A Num. Occ. Structure B Total Num. Occ. Total Carbon 8 x 0. 08333 = 0. 6666 4 x 0. 1666 = 0. 6666 Oxygen 4 x 0. 08333 = 0. 3333 1 x 0. 1666 = 0. 1666 2 x 0. 5(0. 1666) = 0. 1666 } 1. 333 carbons } 0. 6666 oxygens 31 symmetry: multiply all atoms by 3 1. 333 x 3 = 4 carbon atoms 0. 6666 x 3 = 2 oxygen atoms

Torsion Angles ? ? ?

Future Work • PEO-Thiourea inclusion compound (already powder cell, could not make single crystal • PEO with different molecular weights

Acknowledgements • Professor Bruce Foxman • Josh Chen