Crystal Structure and Crystallography of Materials Chapter 3

![1. Cs. Cl Crystal Structure: (110) projection [112] A F E D C B 1. Cs. Cl Crystal Structure: (110) projection [112] A F E D C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-3.jpg)
![2. Na. Cl Crystal Structure (Rock-salt structure): [111] A C B A C B 2. Na. Cl Crystal Structure (Rock-salt structure): [111] A C B A C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-5.jpg)
![4. Zn. S (Zinc Blende) Crystal Structure (Sphalerite): [112] direction (110) projection [111] direction 4. Zn. S (Zinc Blende) Crystal Structure (Sphalerite): [112] direction (110) projection [111] direction](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-9.jpg)
![6. Diamond Crystal Structure: [110] projection [110] Stacking sequence : AABBCC… C C B 6. Diamond Crystal Structure: [110] projection [110] Stacking sequence : AABBCC… C C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-15.jpg)
- Slides: 30

Crystal Structure and Crystallography of Materials Chapter 3: Covalent and Ionic Structures

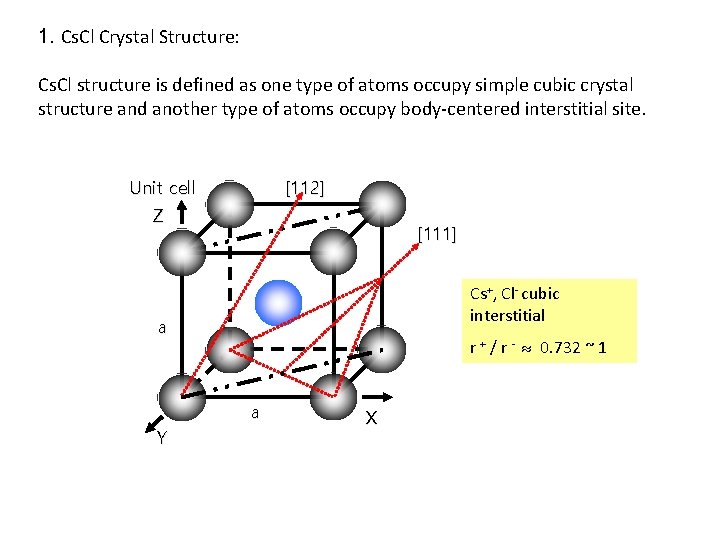
1. Cs. Cl Crystal Structure: Cs. Cl structure is defined as one type of atoms occupy simple cubic crystal structure and another type of atoms occupy body-centered interstitial site. Unit cell [112] Z [111] Cs+, Cl- cubic interstitial a r + / r - 0. 732 ~ 1 a Y X
![1 Cs Cl Crystal Structure 110 projection 112 A F E D C B 1. Cs. Cl Crystal Structure: (110) projection [112] A F E D C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-3.jpg)
1. Cs. Cl Crystal Structure: (110) projection [112] A F E D C B A • Over 400 phases belong to this type such as • Ex. Ag. Cd, Ag. Mg, Cu. Be, Cu. Zn, Cs. Br, Cs. I etc. • Not a BCC structure • Stacking sequence : A B C D E F A B C E D F …

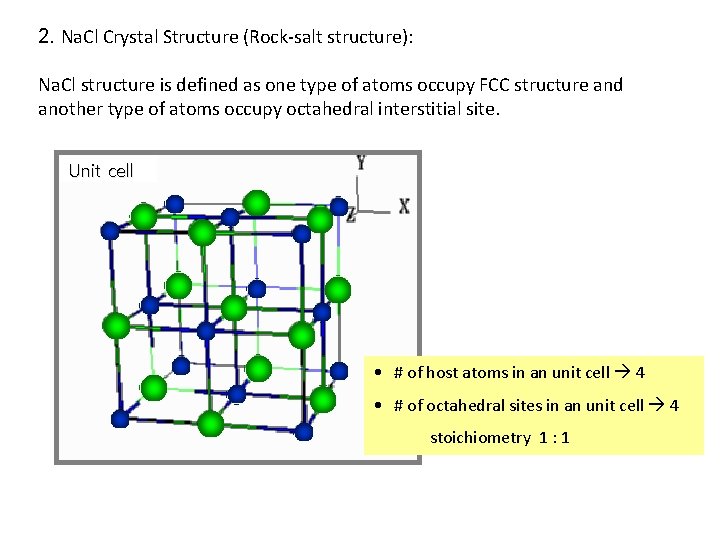
2. Na. Cl Crystal Structure (Rock-salt structure): Na. Cl structure is defined as one type of atoms occupy FCC structure and another type of atoms occupy octahedral interstitial site. Unit cell • # of host atoms in an unit cell 4 • # of octahedral sites in an unit cell 4 stoichiometry 1 : 1
![2 Na Cl Crystal Structure Rocksalt structure 111 A C B A C B 2. Na. Cl Crystal Structure (Rock-salt structure): [111] A C B A C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-5.jpg)
2. Na. Cl Crystal Structure (Rock-salt structure): [111] A C B A C B A (110) projection Stacking sequence : A B C …

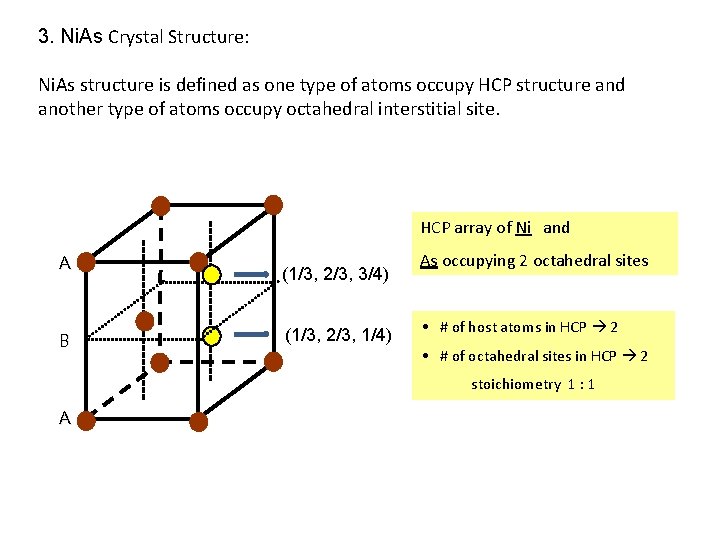
3. Ni. As Crystal Structure: Ni. As structure is defined as one type of atoms occupy HCP structure and another type of atoms occupy octahedral interstitial site. HCP array of Ni and A B (1/3, 2/3, 3/4) (1/3, 2/3, 1/4) As occupying 2 octahedral sites • # of host atoms in HCP 2 • # of octahedral sites in HCP 2 stoichiometry 1 : 1 A

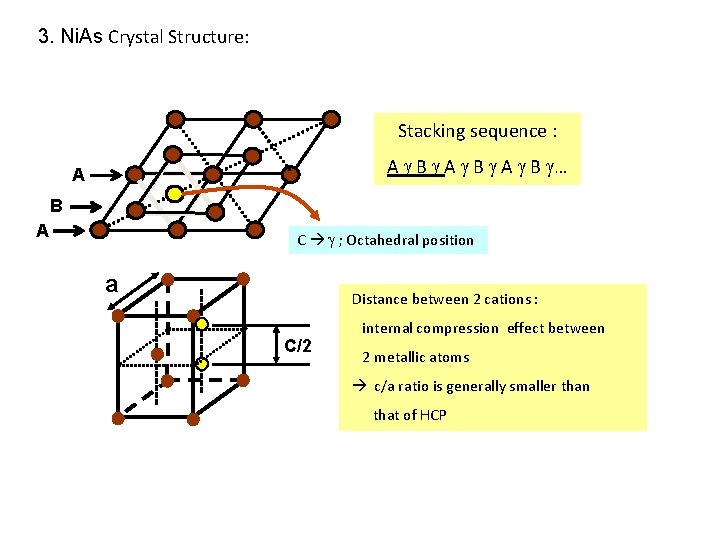
3. Ni. As Crystal Structure: Stacking sequence : A B … A B A C ; Octahedral position a Distance between 2 cations : C/2 internal compression effect between 2 metallic atoms c/a ratio is generally smaller than that of HCP

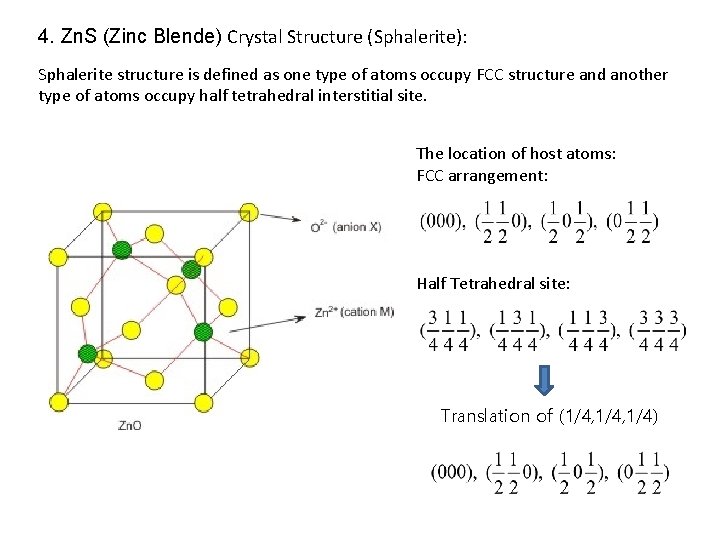
4. Zn. S (Zinc Blende) Crystal Structure (Sphalerite): Sphalerite structure is defined as one type of atoms occupy FCC structure and another type of atoms occupy half tetrahedral interstitial site. The location of host atoms: FCC arrangement: Half Tetrahedral site: Translation of (1/4, 1/4)
![4 Zn S Zinc Blende Crystal Structure Sphalerite 112 direction 110 projection 111 direction 4. Zn. S (Zinc Blende) Crystal Structure (Sphalerite): [112] direction (110) projection [111] direction](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-9.jpg)
4. Zn. S (Zinc Blende) Crystal Structure (Sphalerite): [112] direction (110) projection [111] direction C Stacking sequence : A B C … B A C B A

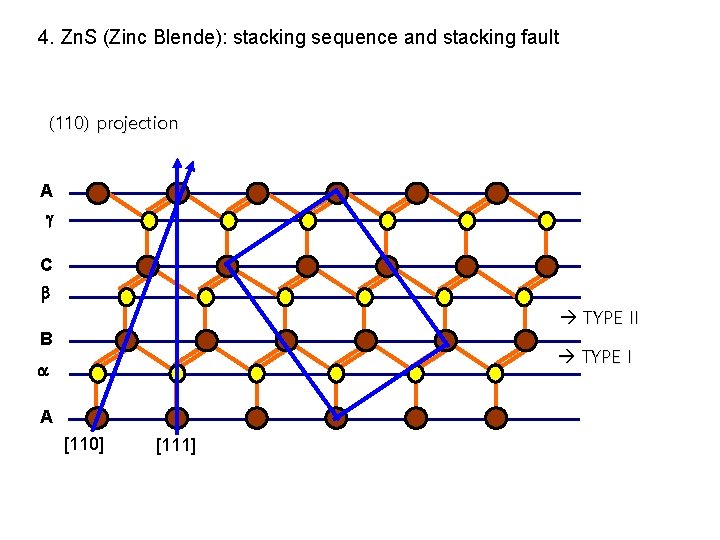
4. Zn. S (Zinc Blende): stacking sequence and stacking fault (110) projection A C TYPE II B TYPE I A [110] [111]

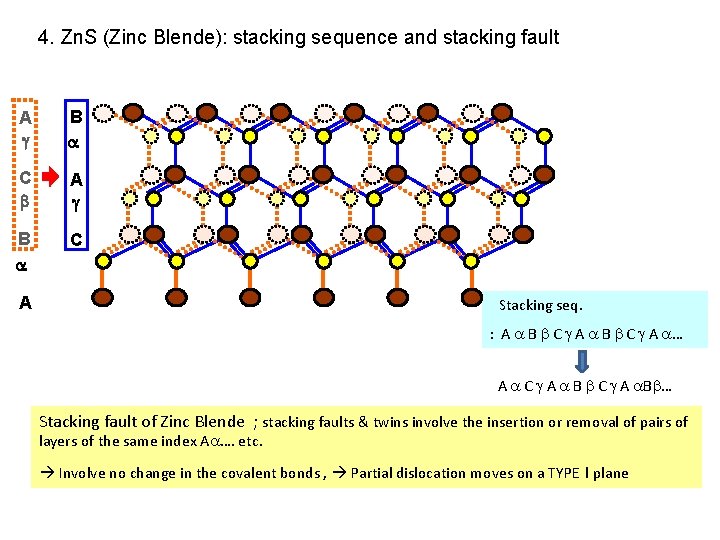
4. Zn. S (Zinc Blende): stacking sequence and stacking fault A B C A Stacking seq. : A B C A … A C A B C A B … Stacking fault of Zinc Blende ; stacking faults & twins involve the insertion or removal of pairs of layers of the same index A …. etc. Involve no change in the covalent bonds , Partial dislocation moves on a TYPE I plane

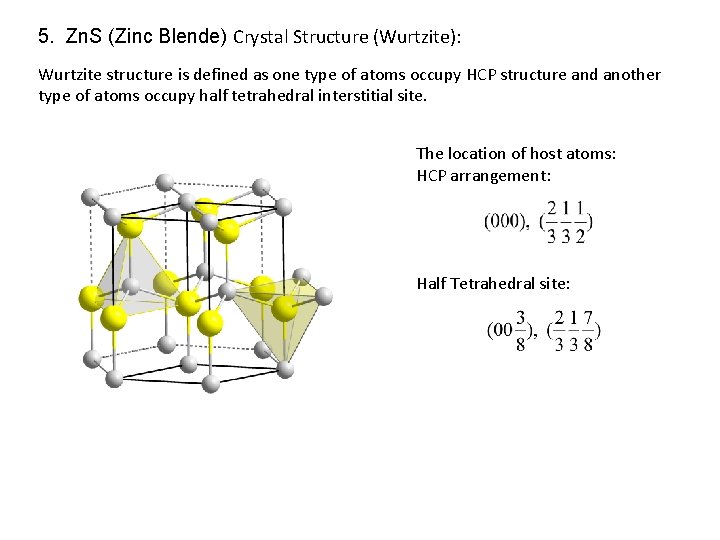
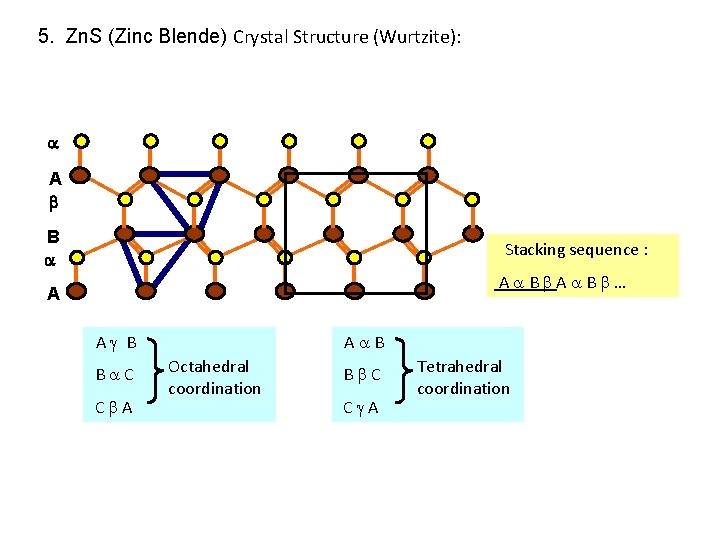
5. Zn. S (Zinc Blende) Crystal Structure (Wurtzite): Wurtzite structure is defined as one type of atoms occupy HCP structure and another type of atoms occupy half tetrahedral interstitial site. The location of host atoms: HCP arrangement: Half Tetrahedral site:

5. Zn. S (Zinc Blende) Crystal Structure (Wurtzite): A B Stacking sequence : A B … A A B B C C A A B Octahedral coordination B C C A Tetrahedral coordination

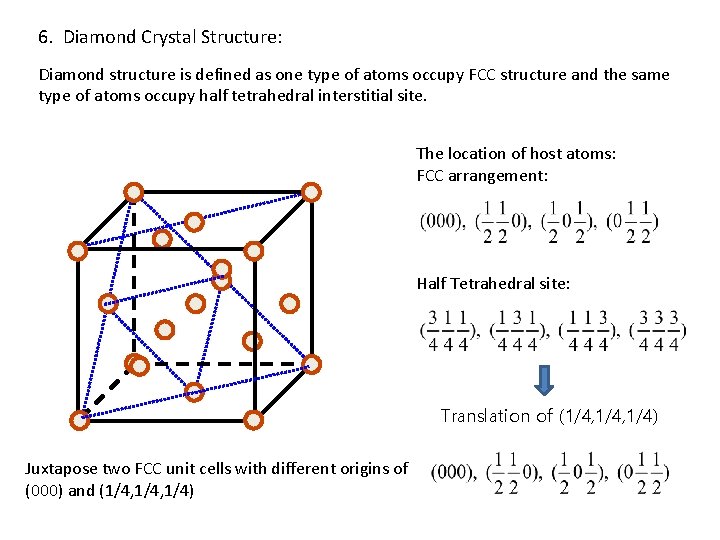
6. Diamond Crystal Structure: Diamond structure is defined as one type of atoms occupy FCC structure and the same type of atoms occupy half tetrahedral interstitial site. The location of host atoms: FCC arrangement: Half Tetrahedral site: Translation of (1/4, 1/4) Juxtapose two FCC unit cells with different origins of (000) and (1/4, 1/4)
![6 Diamond Crystal Structure 110 projection 110 Stacking sequence AABBCC C C B 6. Diamond Crystal Structure: [110] projection [110] Stacking sequence : AABBCC… C C B](https://slidetodoc.com/presentation_image/574c2e06f68f59d66ec150c4fe2c2d0a/image-15.jpg)
6. Diamond Crystal Structure: [110] projection [110] Stacking sequence : AABBCC… C C B Intrinsic stacking fault : AABBCCAA B A BB missing Extrinsic stacking fault : AABBCCAACCBBCC A CC inserting [110]

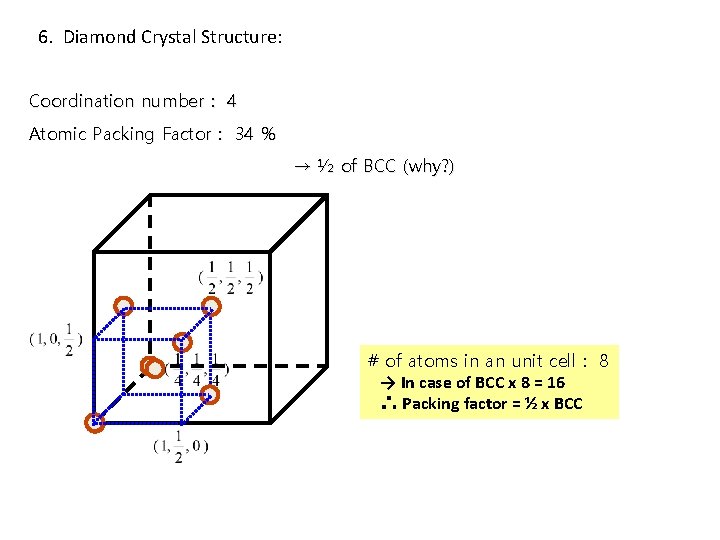
6. Diamond Crystal Structure: Coordination number : 4 Atomic Packing Factor : 34 % → ½ of BCC (why? ) # of atoms in an unit cell : 8 → In case of BCC x 8 = 16 ∴ Packing factor = ½ x BCC

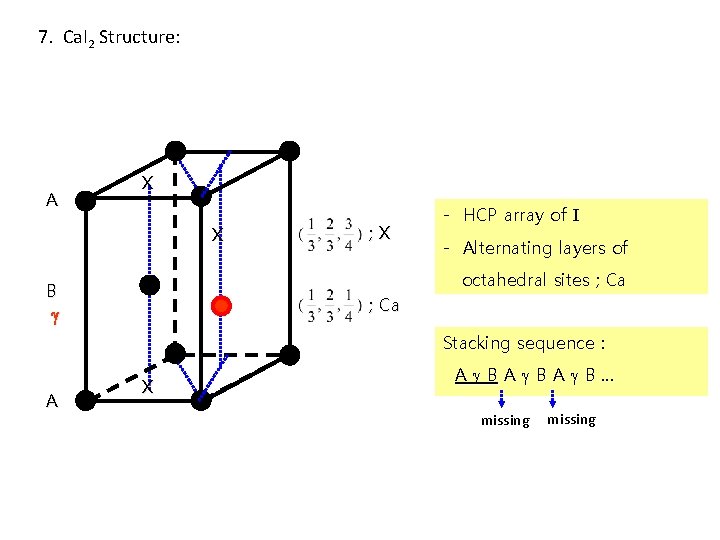
7. Ca. I 2 Structure: A X X ; X - HCP array of I - Alternating layers of octahedral sites ; Ca B ; Ca Stacking sequence : A X A BA BA B… missing

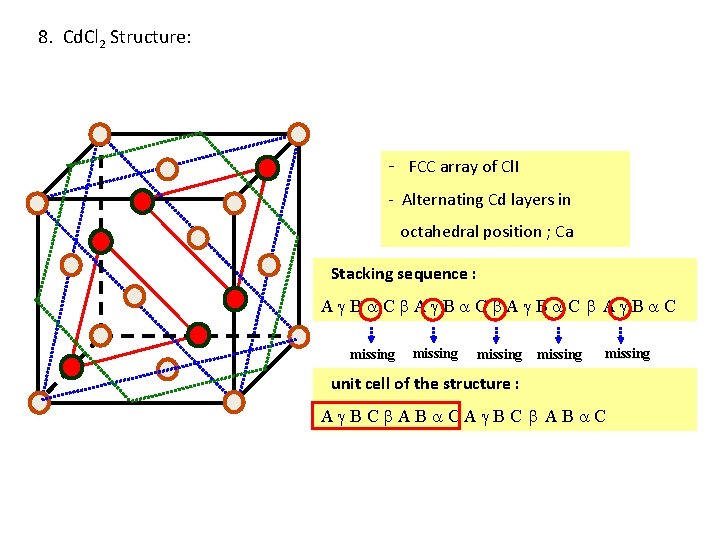
8. Cd. Cl 2 Structure: - FCC array of Cl. I - Alternating Cd layers in octahedral position ; Ca Stacking sequence : A B C missing missing unit cell of the structure : A BC AB CA BC AB C

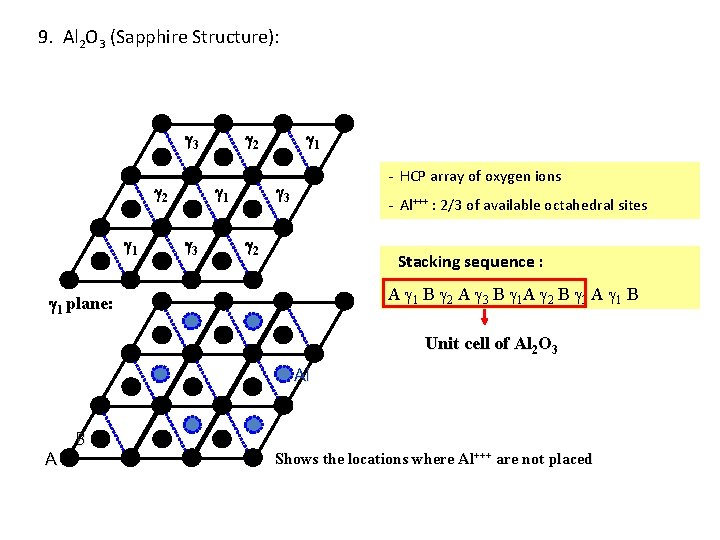
9. Al 2 O 3 (Sapphire Structure): 3 2 1 3 1 - HCP array of oxygen ions 3 - Al+++ : 2/3 of available octahedral sites 2 Stacking sequence : A 1 B 2 A 3 B 1 A 2 B 3 A 1 B 1 plane: Unit cell of Al 2 O 3 Al A B Shows the locations where Al+++ are not placed

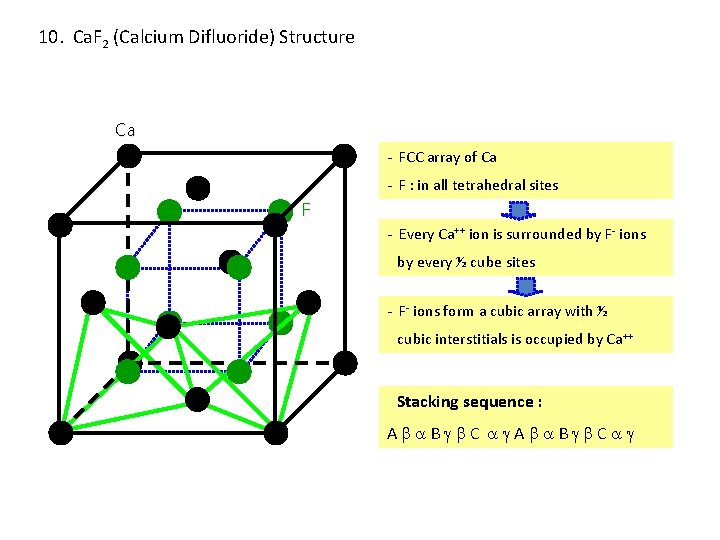
10. Ca. F 2 (Calcium Difluoride) Structure Ca - FCC array of Ca - F : in all tetrahedral sites F - Every Ca++ ion is surrounded by F- ions by every ½ cube sites - F- ions form a cubic array with ½ cubic interstitials is occupied by Ca++ Stacking sequence : A B C

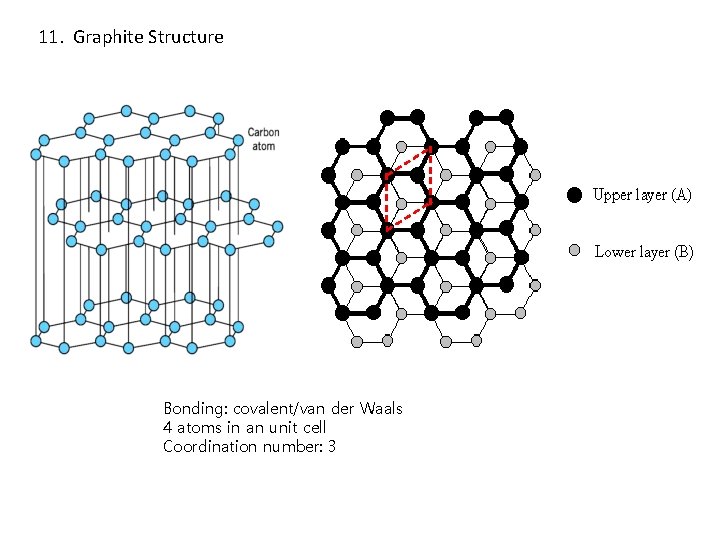
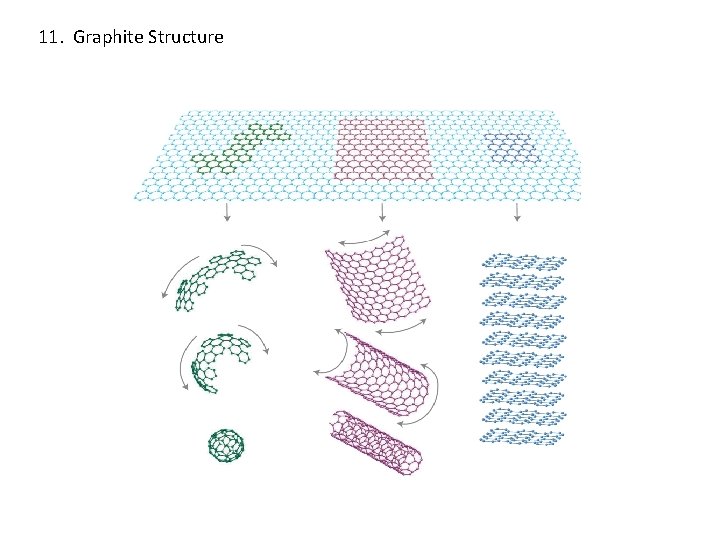
11. Graphite Structure Bonding: covalent/van der Waals 4 atoms in an unit cell Coordination number: 3

11. Graphite Structure

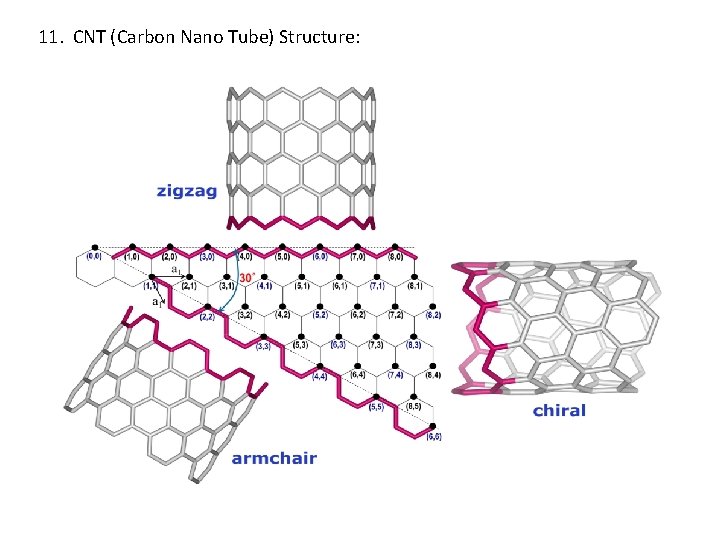
11. CNT (Carbon Nano Tube) Structure:

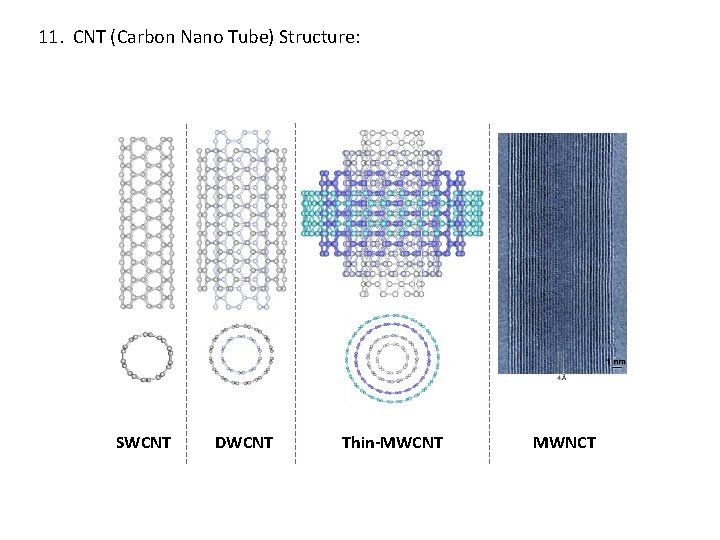
11. CNT (Carbon Nano Tube) Structure: SWCNT DWCNT Thin-MWCNT MWNCT

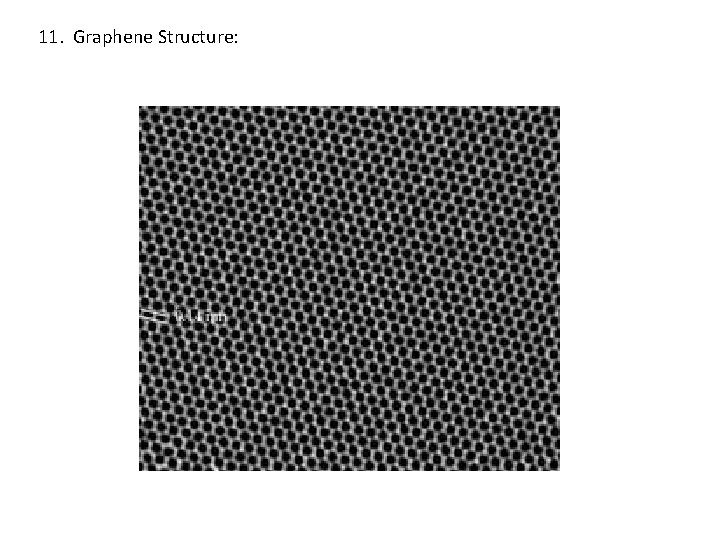
11. Graphene Structure:

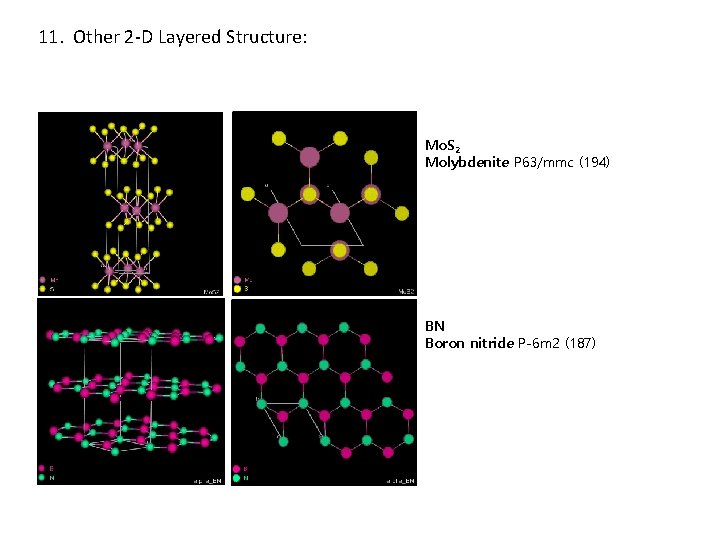
11. Other 2 -D Layered Structure: Mo. S 2 Molybdenite P 63/mmc (194) BN Boron nitride P-6 m 2 (187)

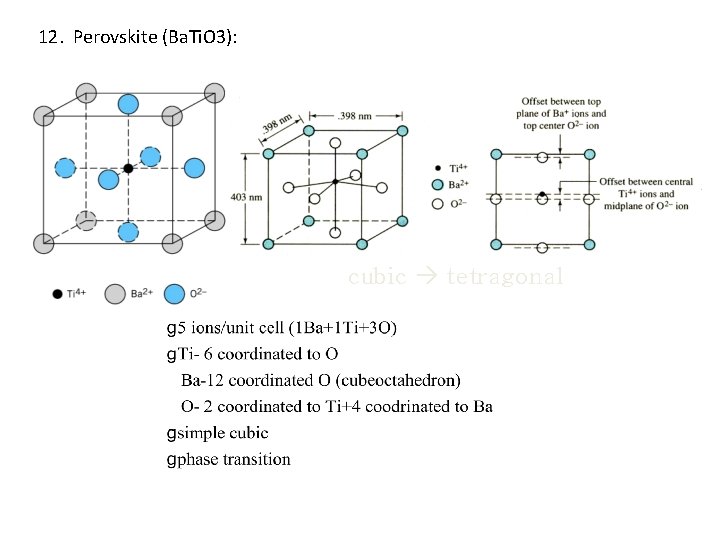
12. Perovskite (Ba. Ti. O 3): cubic tetragonal

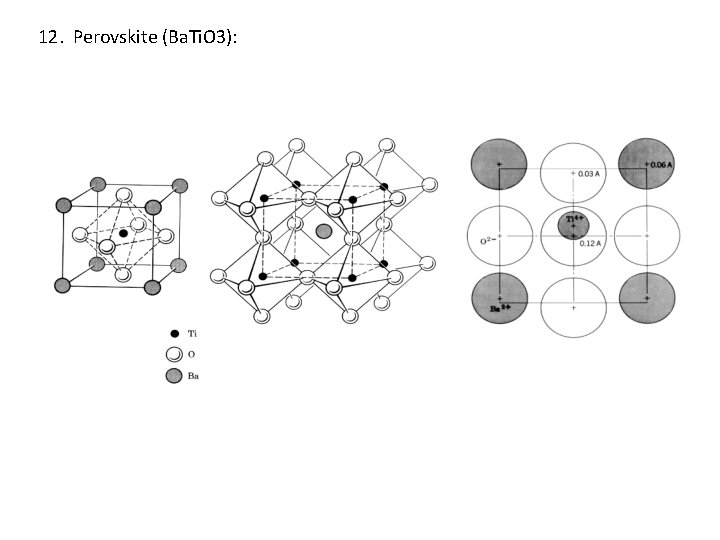
12. Perovskite (Ba. Ti. O 3):

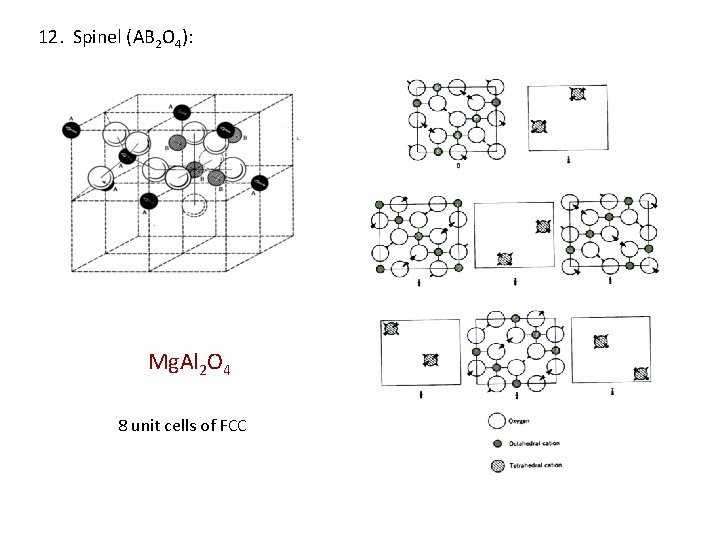
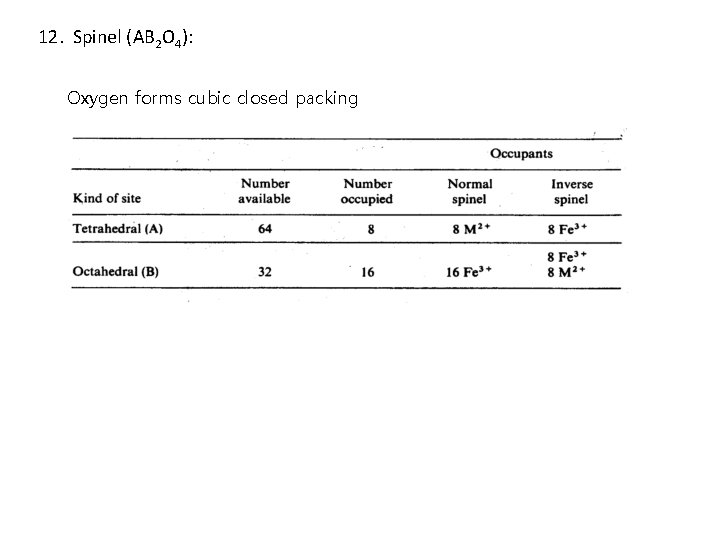
12. Spinel (AB 2 O 4): Mg. Al 2 O 4 8 unit cells of FCC

12. Spinel (AB 2 O 4): Oxygen forms cubic closed packing