Crystal Growth How do single crystals differ from








- Slides: 11

Crystal Growth • • • How do single crystals differ from polycrystalline samples? Atomic arrays that are periodic in three dimensions, with repeated distances are called single crystals. Single crystal specimens maintain translational symmetry over macroscopic distances (crystal dimensions are typically 0. 1 mm – 10 cm). Why would one go to the effort of growing a single crystal? Structure determination and intrinsic property measurements are preferably, sometimes exclusively, carried out on single crystals. For certain applications, most notably those which rely on optical and/or electronic properties (laser crystals, semiconductors, etc. ), single crystals are necessary. There would be no electronic industry, no photonic industry, no fiber optic communications without single crystal materials. Growth of single crystals and their characterization towards device fabrication have assumed great impetus (motivation) due to their importance for both academic as well as applied research.

Some uses of Crystals • Nonlinear optical crystals are very important for laser frequency conversion. For example • Potassium dihydrogen phosphate (KDP) is suitable for higher harmonic generation of huge laser systems for fusion experiments because it can be grown to larger sizes and also KDP has a high laser damage threshold. • Potassium titanyl phosphate (KTP) is a useful nonlinear optical crystal to get efficient green light by the frequency doubling of Nd: YAG laser. It has high optical nonlinearity, large temperature and angular allowance and it is non hygroscopic and mechanically hard.


METHODS OF Crystal Growth • It is clearly more difficult to prepare single crystal than poly-crystalline material and extra effort is justified because of the outstanding advantages of single crystals. • Growth of crystal ranges from a small inexpensive technique to a complex sophisticated expensive process and crystallization time ranges from minutes, hours, days and to months.

Ø Single crystals may be produced by the transport of crystal constituents in the solid, liquid or vapour phase. On the basis of this, crystal growth may be classified into three categories as follows, Solid Growth - Solid-to-Solid phase transformation Liquid Growth - Liquid to Solid phase transformation Vapour Growthtransformation Vapour to Solid phase

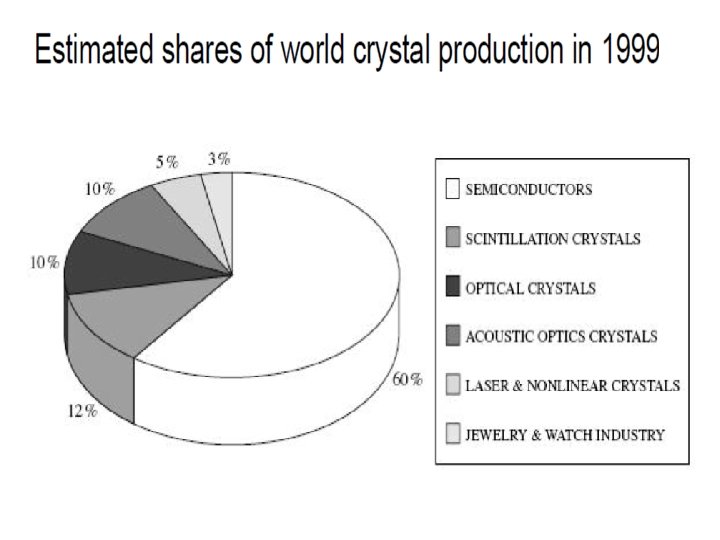
Ø An efficient process is the one, which produces crystals adequate for their use at minimum cost. Ø Better choice of the growth method is essential because it suggests the possible impurity and other defect concentrations. Ø Choosing the best method to grow a given material depends on material characteristics. Ø Liquid growth includes both melt and solution growth. Ø A survey of the methods of growth suggests that Ø Almost 80% of the single crystals are grown from the melt Ø Roughly 5% from vapour, 5% from low temperature solution, 5% from high temperature solution, and 3% from the solid and only 2% by hydrothermal methods.

What factors control the size and purity of single crystals? Nucleation and Growth. If nucleation rates are slow and growth is rapid, large crystals will result. On the other hand, if nucleation is rapid, relative to growth, small crystals or even polycrystalline samples will result. What can be done to increase the growth rates? In order to attain the rapid growth rates needed to grow macroscopic crystals, diffusion coefficients must be large. Hence, crystal growth typically occurs via formation of a solid from another state of matter : (a) Liquid (Melt) àSolid (Freezing) (b) Gas (Vapor) à Solid (Condensation) (c) Solution à Solid (Precipitation) • It should be noted that defect concentrations tend to increase as the growth rate increases. Consequently the highest quality crystals need to be grown slowly.

What can be done to limit the number of nucleation sites? Several techniques are used separately or in combination to induce nucleation of the solid phase at a slow and controlled rate : (a) Slow Cooling of Melts (b) Temperature Gradients (c) Introduction of Seed Crystals

Slow cooling of the melt • With congruently melting materials (those which maintain the same composition on melting), one simply melts a mixture of the desired composition then cools slowly (typically 2 -10 °C/h) through the melting point. • More difficult with incongruently melting materials, knowledge of the phase diagram is needed. • Very often, the phase diagram is not known. Consequently, there is no guarantee that crystals will have the intended stoichiometry. • Molten salt fluxes are often used to facilitate crystal growth in systems where melting points are very high and/or incongruent melting occurs. • Crystals grown in this way are often rather small. Thus, this method is frequently used in research, but usually not appropriate for applications where large crystals are needed.

Growth from melt Melt growth is undoubtedly the best method for growing large single crystals of high perfection relatively rapidly and has been extensively used for metals, semiconductors ionic crystals and a few organic compounds. In semiconductors and laser host crystals, impurities (dopants) can be deliberately added and homogeneously dispersed in a large percentage of the grown crystals. The method has been developed largely in electronics, optics and synthetic gemstone industries. Melt growth requires that the material melts congruently (i. e. , it does not decompose below or near its melting point) and has Q 1` a manageable vapour pressure at its melting point. The rate of growth of crystals by this method is mostly used in commercial purposes. However, many hydrates and anhydrated salts, organic salts and virtually all biological materials cannot be grown from this method. The melt grown can be further subdivided into various techniques.

Congruent and Incongruent Melting in Binary and Ternary Systems • The thermal behavior of intermediate compounds is of three basic types: congruent melting, incongruent melting, or dissociation. • An intermediate compound is a combination of the two end members of a binary or ternary phase diagram that forms a different component between the two solids. • Congruency of melting is important in the determination of phase analysis diagrams and in drawing crystallization paths.