Crystal Growth and Structural Defects Dr Stephen Crabtree

Crystal Growth and Structural Defects Dr. Stephen Crabtree Sept. 28, 2018
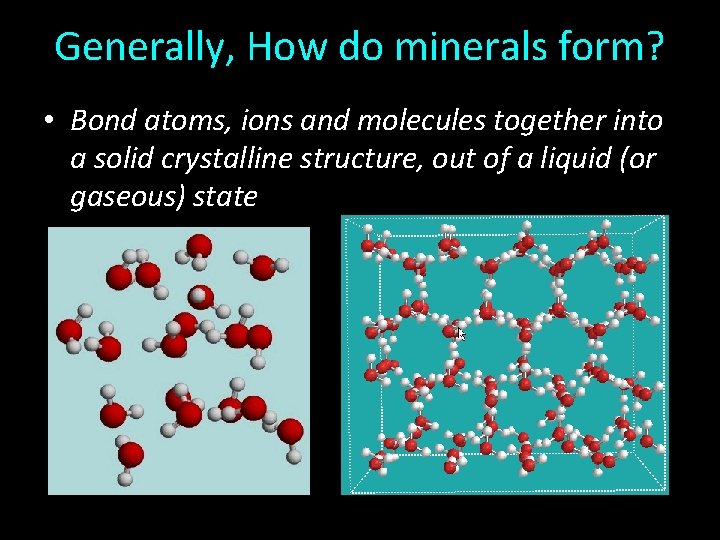
Generally, How do minerals form? • Bond atoms, ions and molecules together into a solid crystalline structure, out of a liquid (or gaseous) state

Generally, How do minerals form? • Three general ways: – Crystallization of a solute from solution • Evaporate away the solvent to increase concentration • Solution becomes oversaturated at lower temperatures • Solution becomes oversaturated at lower pressures

Generally, How do minerals form? • Three general ways: – Crystallization of/from a melt • Link ions/molecules within the melt to each other • Cool the melt in order to: 1. Slow thermal vibrations of atoms 2. Relatively increase the attractive forces between atoms

Generally, How do minerals form? • Three general ways: – Crystallization from an oversaturated vapor • Link ions and atoms from the vapor • Typically linking only particular elements or molecules from the vapor, not the whole vapor
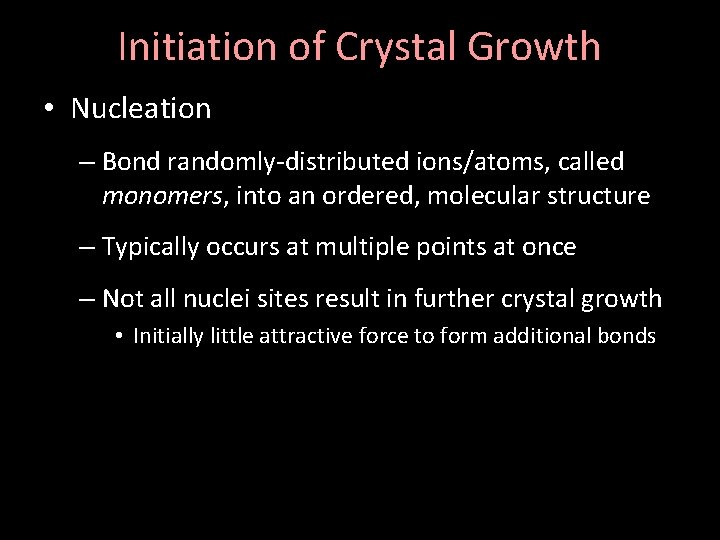
Initiation of Crystal Growth • Nucleation – Bond randomly-distributed ions/atoms, called monomers, into an ordered, molecular structure – Typically occurs at multiple points at once – Not all nuclei sites result in further crystal growth • Initially little attractive force to form additional bonds
Initiation of Crystal Growth
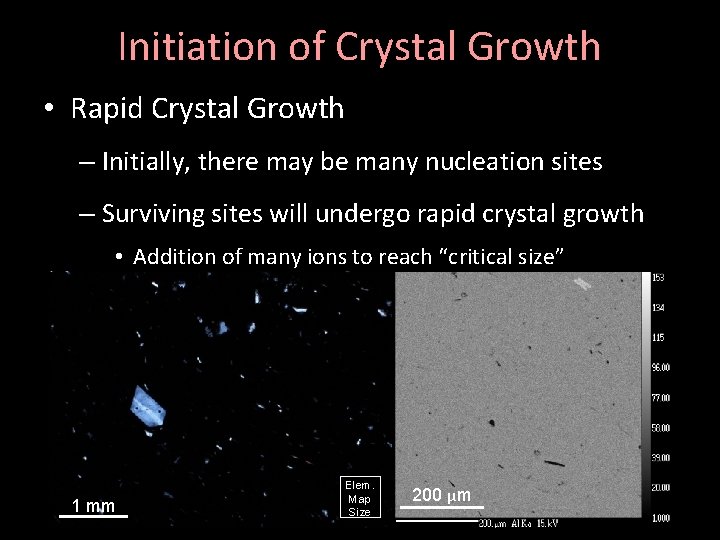
Initiation of Crystal Growth • Rapid Crystal Growth – Initially, there may be many nucleation sites – Surviving sites will undergo rapid crystal growth • Addition of many ions to reach “critical size” TAN-13 1 mm Elem. Map Size 200 µm

Initiation of Crystal Growth • Rapid Crystal Growth – Initially, there may be many nucleation sites – Surviving sites will undergo rapid crystal growth • Addition of many ions to reach “critical size” TAN-13 1 mm Elem. Map Size 200 µm
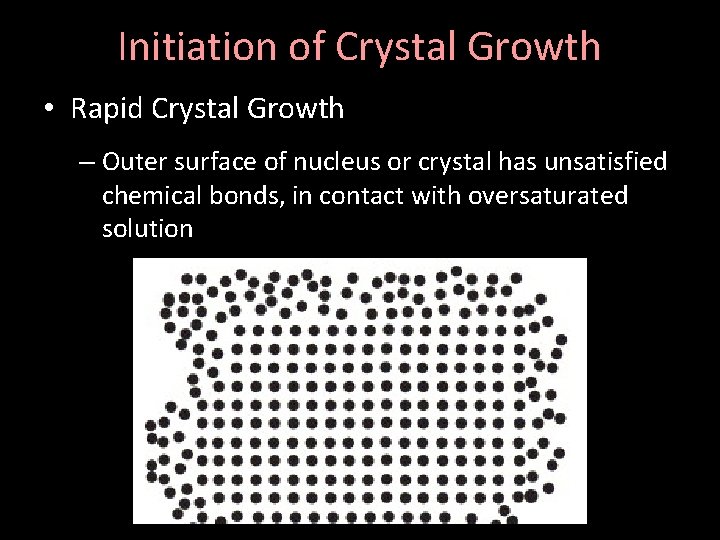
Initiation of Crystal Growth • Rapid Crystal Growth – Outer surface of nucleus or crystal has unsatisfied chemical bonds, in contact with oversaturated solution
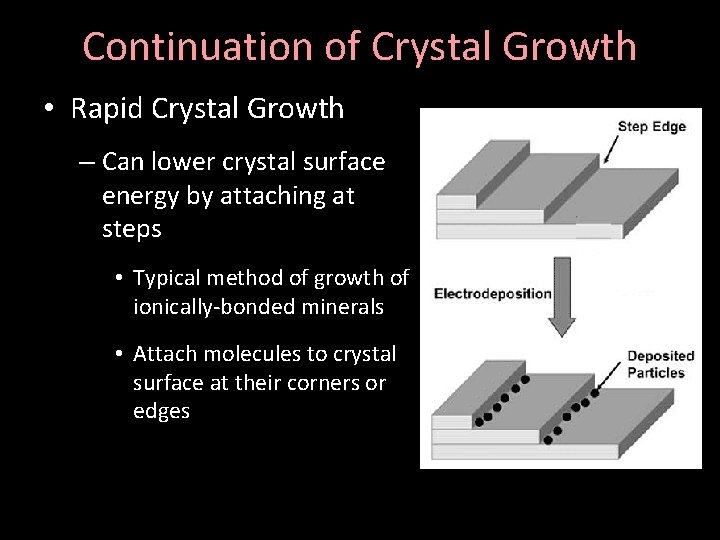
Continuation of Crystal Growth • Rapid Crystal Growth – Can lower crystal surface energy by attaching at steps • Typical method of growth of ionically-bonded minerals • Attach molecules to crystal surface at their corners or edges
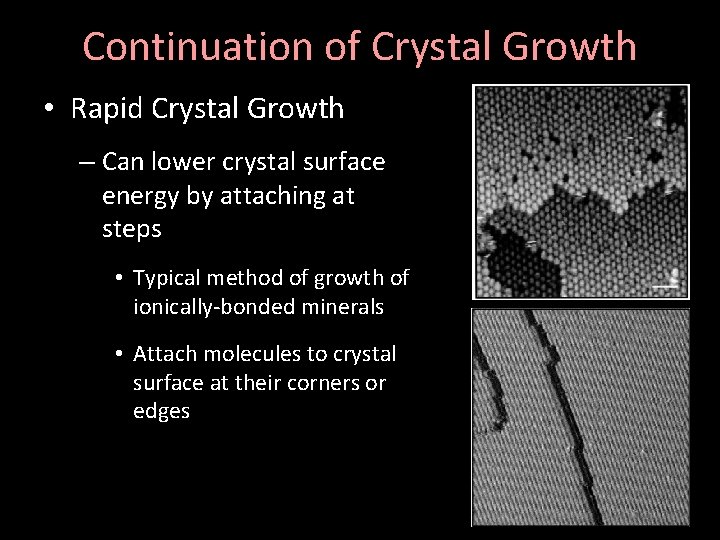
Continuation of Crystal Growth • Rapid Crystal Growth – Can lower crystal surface energy by attaching at steps • Typical method of growth of ionically-bonded minerals • Attach molecules to crystal surface at their corners or edges

Continuation of Crystal Growth • Rapid Crystal Growth – Can lower crystal surface energy by attaching at steps • Typical method of growth of ionically-bonded minerals • Attach molecules to crystal surface at their corners or edges • May lead to the dendritic crystal growth

Continuation of Crystal Growth • Rapid Crystal Growth – Can lower crystal surface energy by attaching in clumps • Attach molecules to crystal surface at their faces • Thought to be common with both ionic and nonionicallybonded minerals • May also provide sites for future step-focused growth
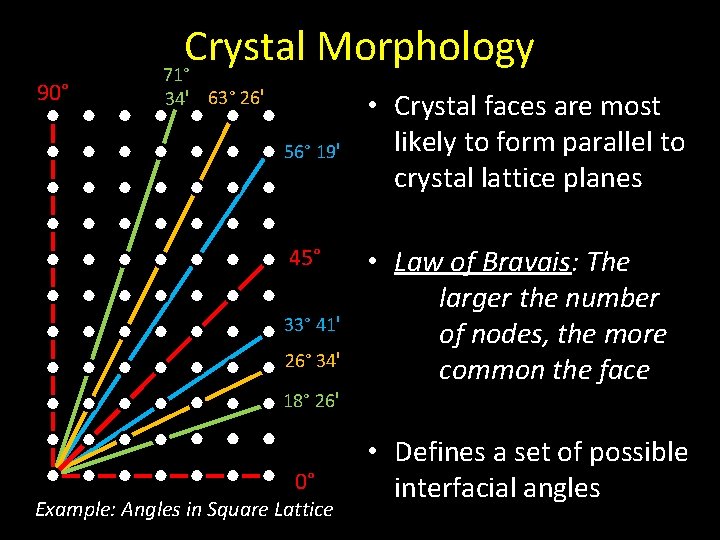
90° Crystal Morphology 71° 34ꞌ 63° 26ꞌ 56° 19ꞌ 45° 33° 41ꞌ 26° 34ꞌ • Crystal faces are most likely to form parallel to crystal lattice planes • Law of Bravais: The larger the number of nodes, the more common the face 18° 26ꞌ 0° Example: Angles in Square Lattice • Defines a set of possible interfacial angles
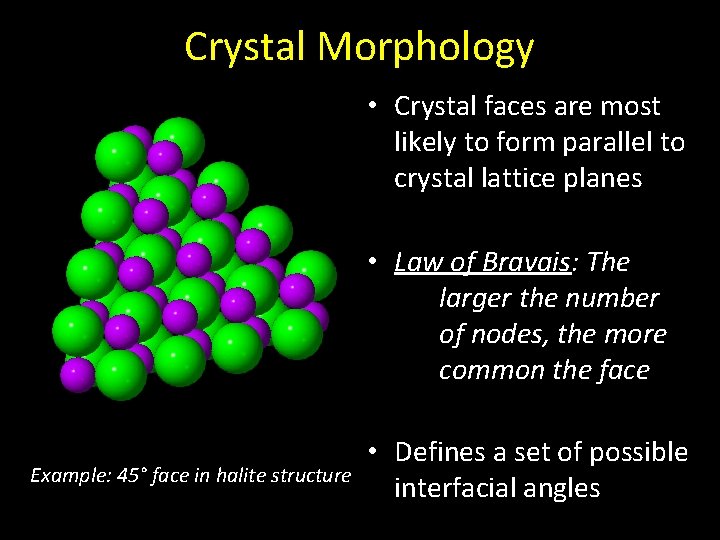
Crystal Morphology • Crystal faces are most likely to form parallel to crystal lattice planes • Law of Bravais: The larger the number of nodes, the more common the face Example: 45° face in halite structure • Defines a set of possible interfacial angles
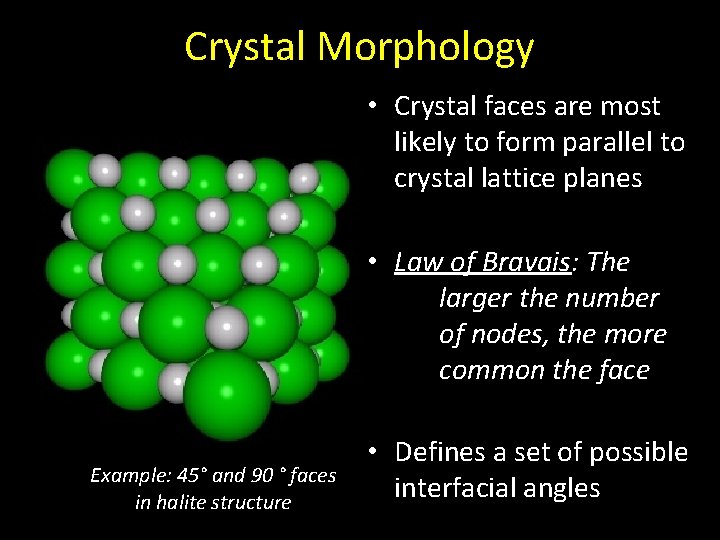
Crystal Morphology • Crystal faces are most likely to form parallel to crystal lattice planes • Law of Bravais: The larger the number of nodes, the more common the face Example: 45° and 90 ° faces in halite structure • Defines a set of possible interfacial angles
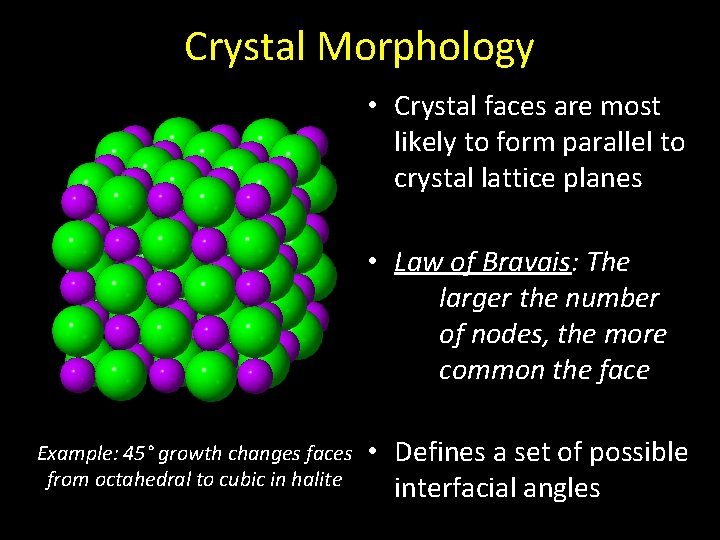
Crystal Morphology • Crystal faces are most likely to form parallel to crystal lattice planes • Law of Bravais: The larger the number of nodes, the more common the face Example: 45° growth changes faces from octahedral to cubic in halite • Defines a set of possible interfacial angles
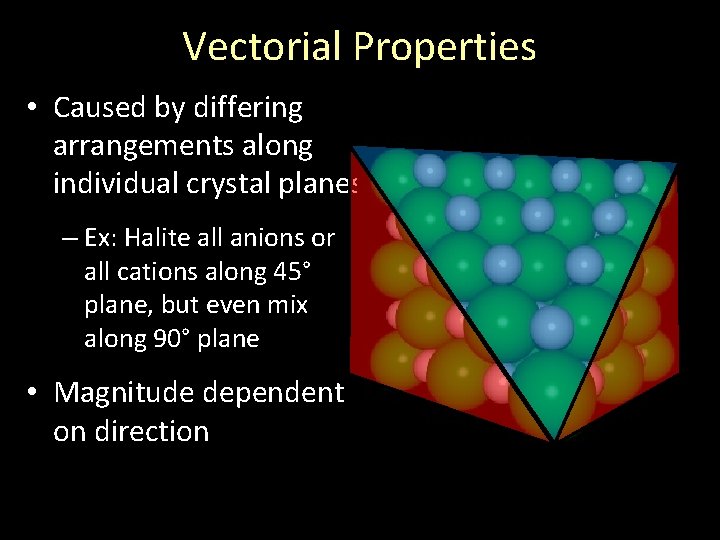
Vectorial Properties • Caused by differing arrangements along individual crystal planes – Ex: Halite all anions or all cations along 45° plane, but even mix along 90° plane • Magnitude dependent on direction

Continuous Vectorial Properties • Vary continuously with direction within a crystal • Example Properties: – Hardness – Conductivity – Thermal Expansion – Speed of Light – Etc…

Discontinuous Vectorial Properties • Only relate to certain definite planes or directions within a crystal • Example Properties: – Growth Rate – Cleavage – Solution Rate – X-Ray Diffraction – Etc…

Discontinuous Vectorial Properties • Only relate to certain definite planes or directions within a crystal • Example Property: – Growth Rate • Higher density of nodes in 0° and 90° planes, most stable • Faster growth in other planes because they require fewer bonding ions 90° 71° 34ꞌ 63° 26ꞌ 56° 19ꞌ 45° 33° 41ꞌ 26° 34ꞌ 18° 26ꞌ 0° May result in dendritic growth

Discontinuous Vectorial Properties • Only relate to certain definite planes or directions within a crystal • Example Property: – Growth Rate • Early, rapid growth, will be along high-energy planes • Slower, more stable planes will lag in growth rate • Rate inversely proportional to node density
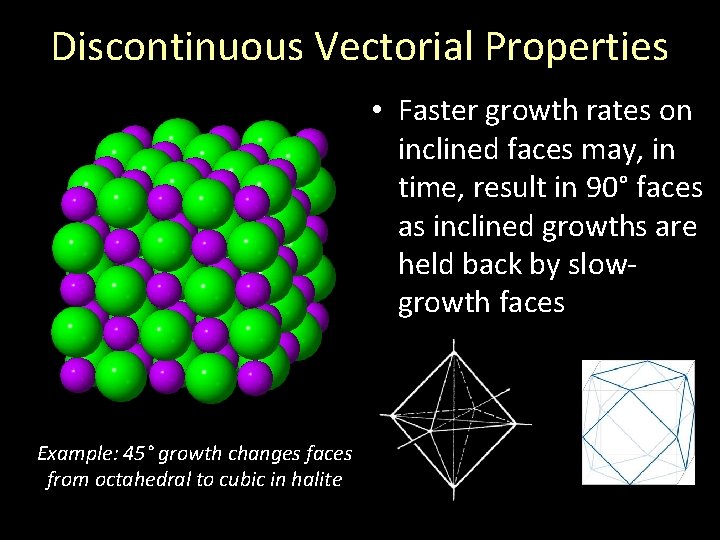
Discontinuous Vectorial Properties • Faster growth rates on inclined faces may, in time, result in 90° faces as inclined growths are held back by slowgrowth faces Example: 45° growth changes faces from octahedral to cubic in halite

Discontinuous Vectorial Properties • Only relate to certain definite planes or directions within a crystal • Example Property: – Cleavage • Found in planes with the weakest bonding energy • Tend to be most denselypopulated and/or widely spaced planes

Ordered Intergrowth of Crystals • Parallel growth – Same mineral, same orientation

Ordered Intergrowth of Crystals • Twinned growth – Same mineral, different orientation along a mirror or rotation plane
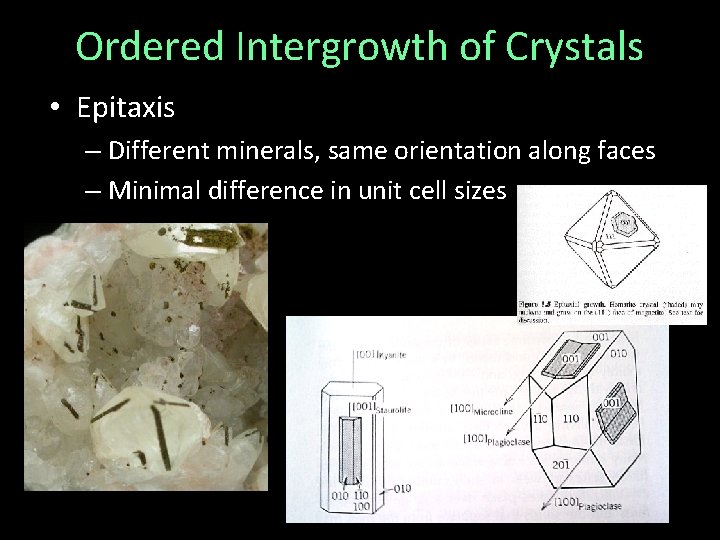
Ordered Intergrowth of Crystals • Epitaxis – Different minerals, same orientation along faces – Minimal difference in unit cell sizes
Structural Defects • Structures tend not to be perfect – High-resolution images show defects on molecular -to-atomic scales • Defects can affect a range of properties – Crystal strength: hardness, tenacity – Conductivity – Deformability – Color – etc.
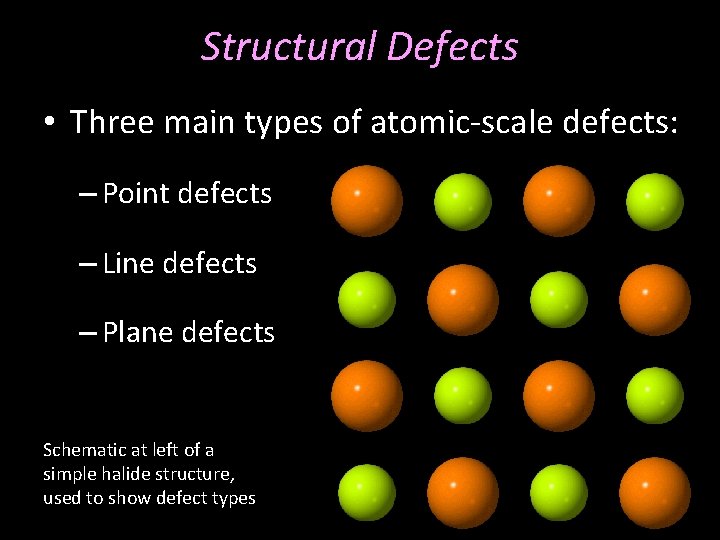
Structural Defects • Three main types of atomic-scale defects: – Point defects – Line defects – Plane defects Schematic at left of a simple halide structure, used to show defect types
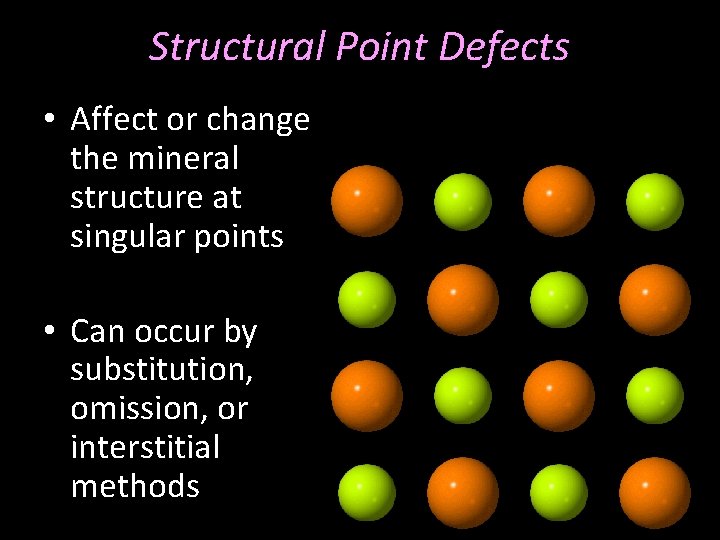
Structural Point Defects • Affect or change the mineral structure at singular points • Can occur by substitution, omission, or interstitial methods
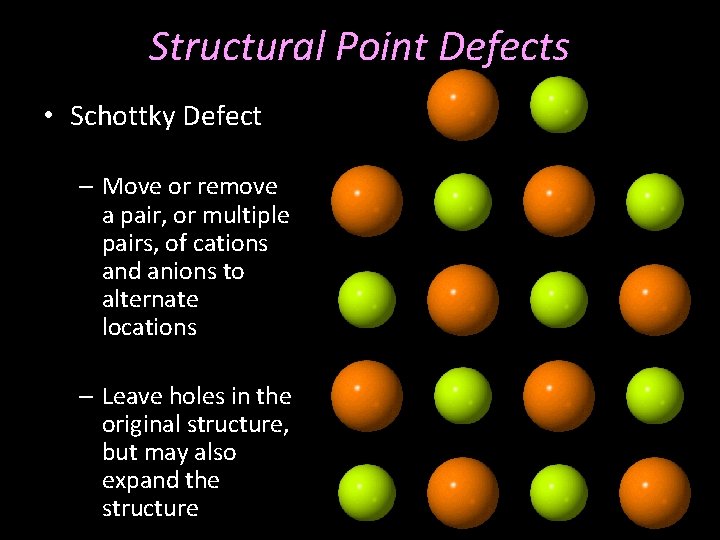
Structural Point Defects • Schottky Defect – Move or remove a pair, or multiple pairs, of cations and anions to alternate locations – Leave holes in the original structure, but may also expand the structure
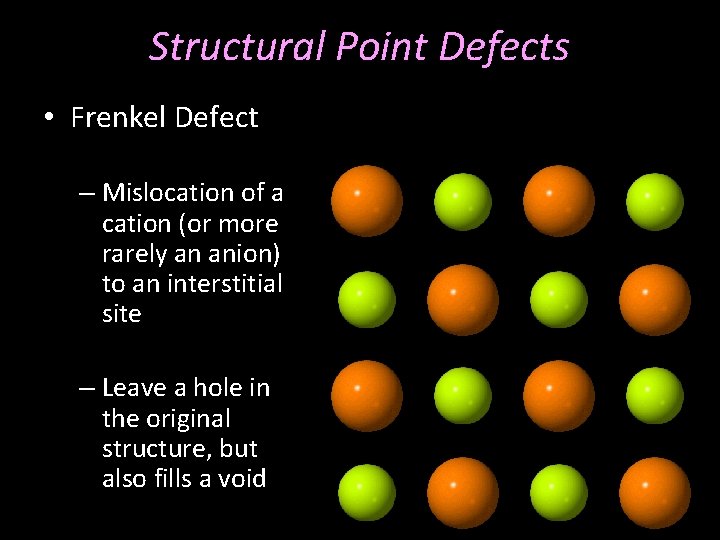
Structural Point Defects • Frenkel Defect – Mislocation of a cation (or more rarely an anion) to an interstitial site – Leave a hole in the original structure, but also fills a void
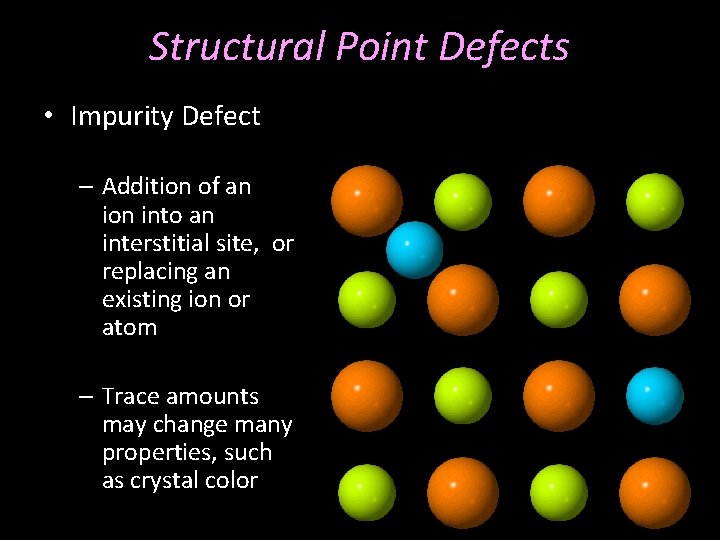
Structural Point Defects • Impurity Defect – Addition of an ion into an interstitial site, or replacing an existing ion or atom – Trace amounts may change many properties, such as crystal color
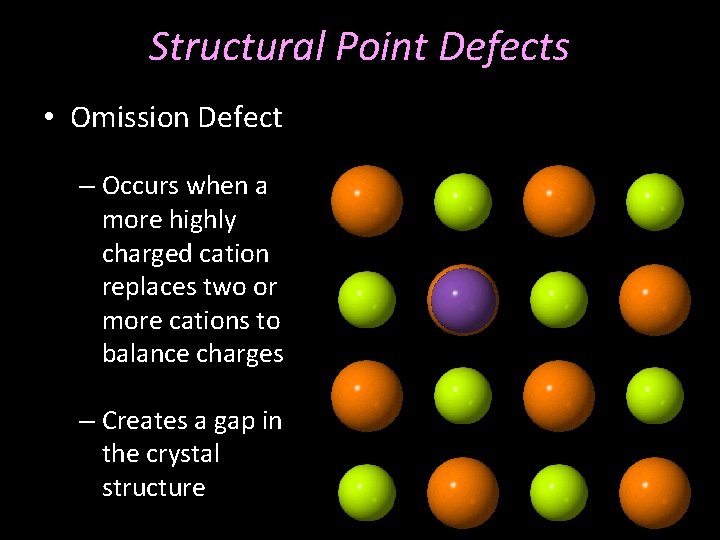
Structural Point Defects • Omission Defect – Occurs when a more highly charged cation replaces two or more cations to balance charges – Creates a gap in the crystal structure
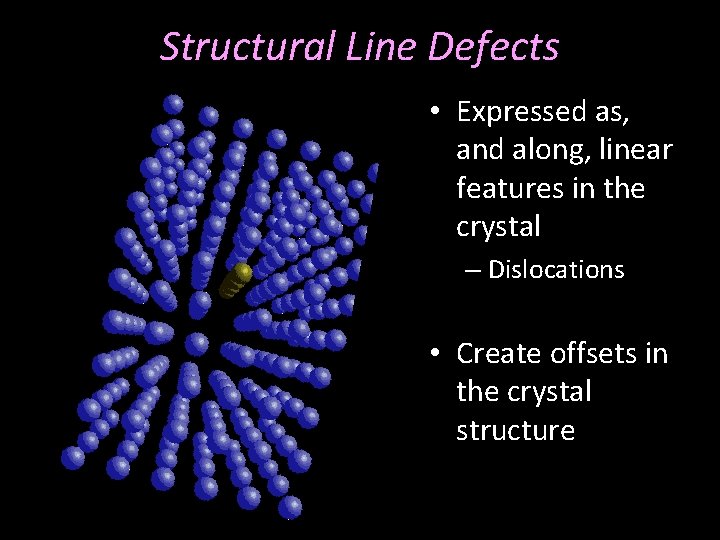
Structural Line Defects • Expressed as, and along, linear features in the crystal – Dislocations • Create offsets in the crystal structure
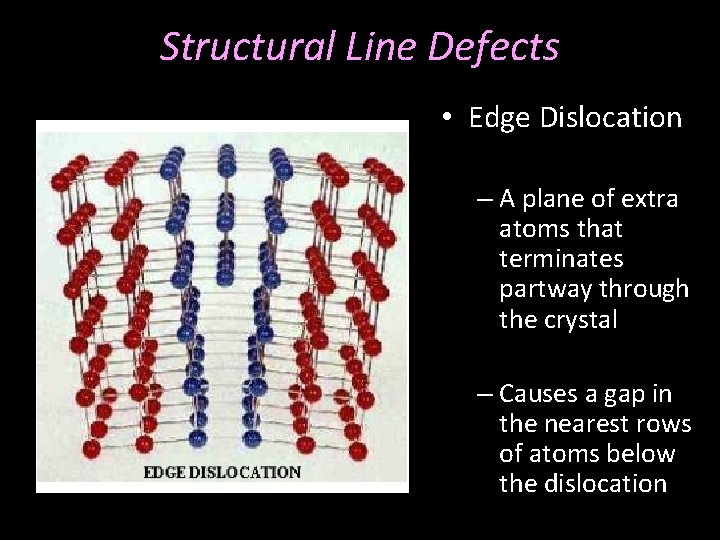
Structural Line Defects • Edge Dislocation – A plane of extra atoms that terminates partway through the crystal – Causes a gap in the nearest rows of atoms below the dislocation
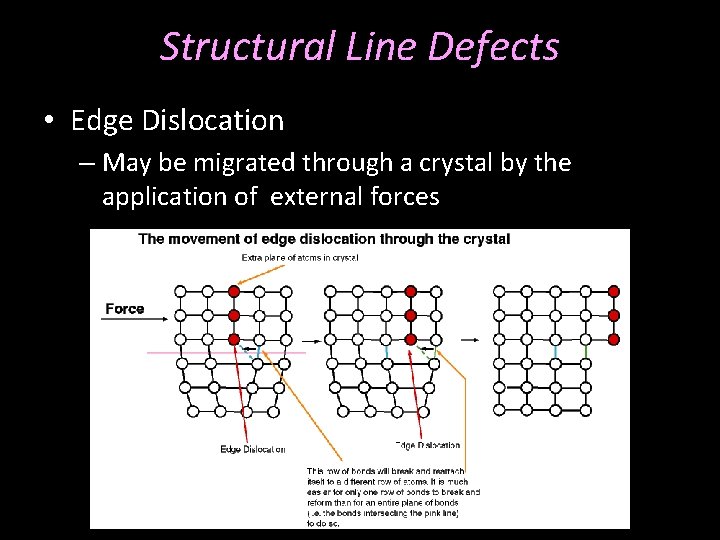
Structural Line Defects • Edge Dislocation – May be migrated through a crystal by the application of external forces
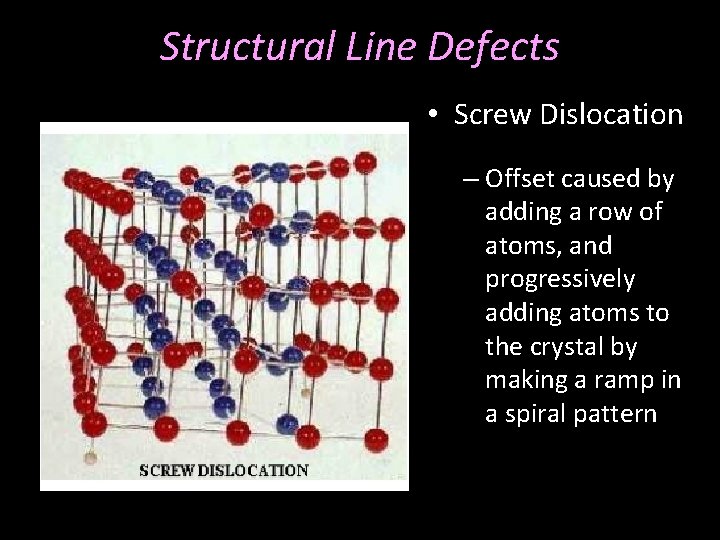
Structural Line Defects • Screw Dislocation – Offset caused by adding a row of atoms, and progressively adding atoms to the crystal by making a ramp in a spiral pattern
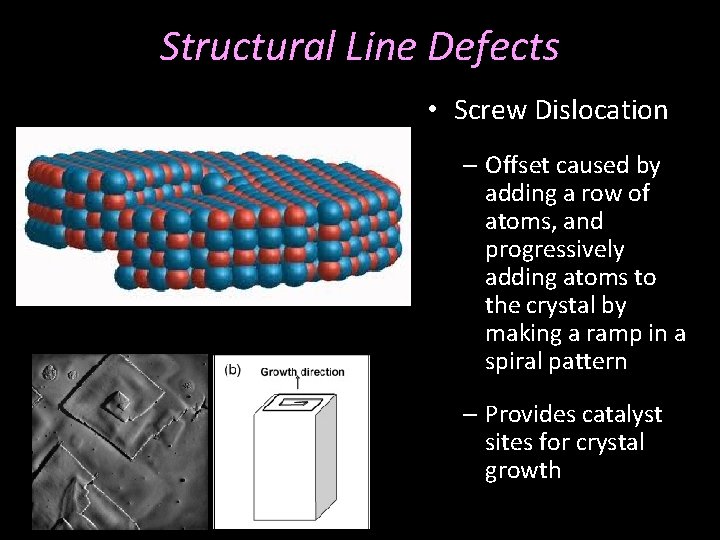
Structural Line Defects • Screw Dislocation – Offset caused by adding a row of atoms, and progressively adding atoms to the crystal by making a ramp in a spiral pattern – Provides catalyst sites for crystal growth
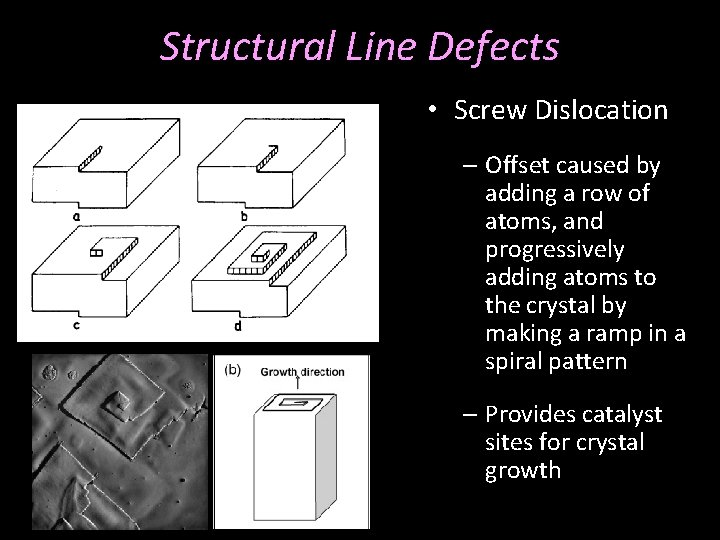
Structural Line Defects • Screw Dislocation – Offset caused by adding a row of atoms, and progressively adding atoms to the crystal by making a ramp in a spiral pattern – Provides catalyst sites for crystal growth
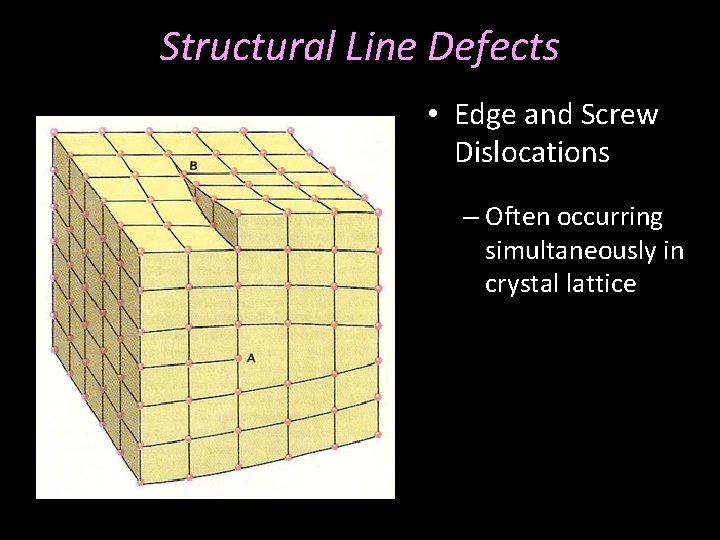
Structural Line Defects • Edge and Screw Dislocations – Often occurring simultaneously in crystal lattice
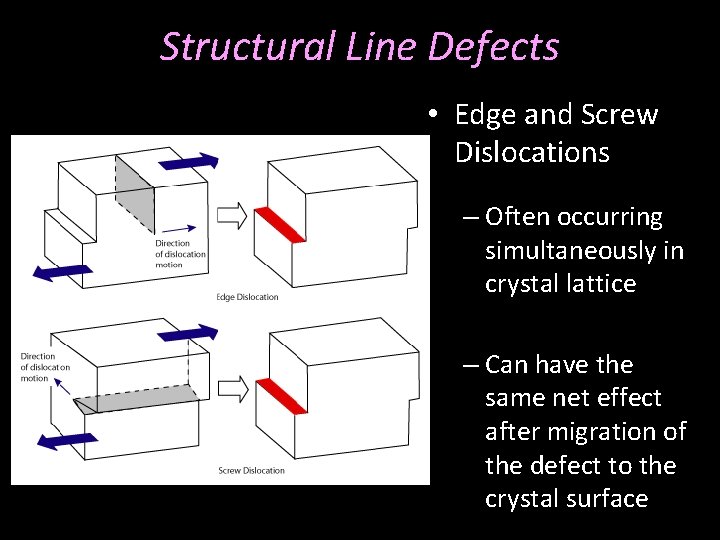
Structural Line Defects • Edge and Screw Dislocations – Often occurring simultaneously in crystal lattice – Can have the same net effect after migration of the defect to the crystal surface
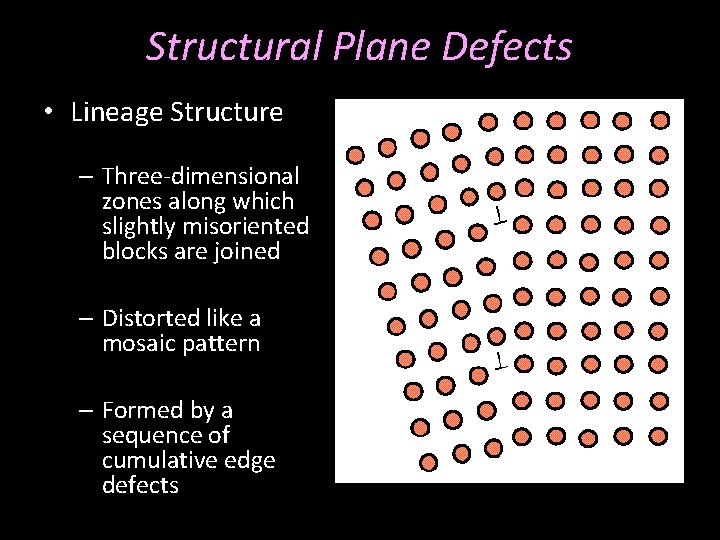
Structural Plane Defects • Lineage Structure – Three-dimensional zones along which slightly misoriented blocks are joined – Distorted like a mosaic pattern – Formed by a sequence of cumulative edge defects
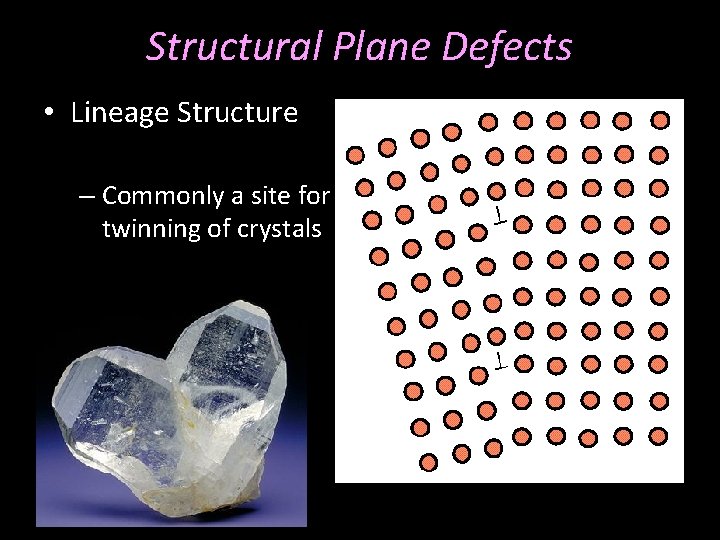
Structural Plane Defects • Lineage Structure – Commonly a site for twinning of crystals
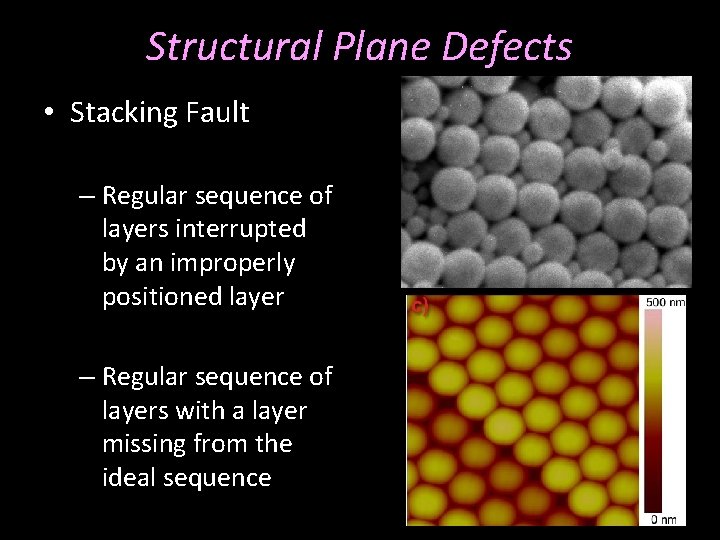
Structural Plane Defects • Stacking Fault – Regular sequence of layers interrupted by an improperly positioned layer – Regular sequence of layers with a layer missing from the ideal sequence

Twinning • A symmetric intergrowth of crystals along or initiated by structural defects • Results from a deviation from perfectly balanced energy of crystal structure internal a
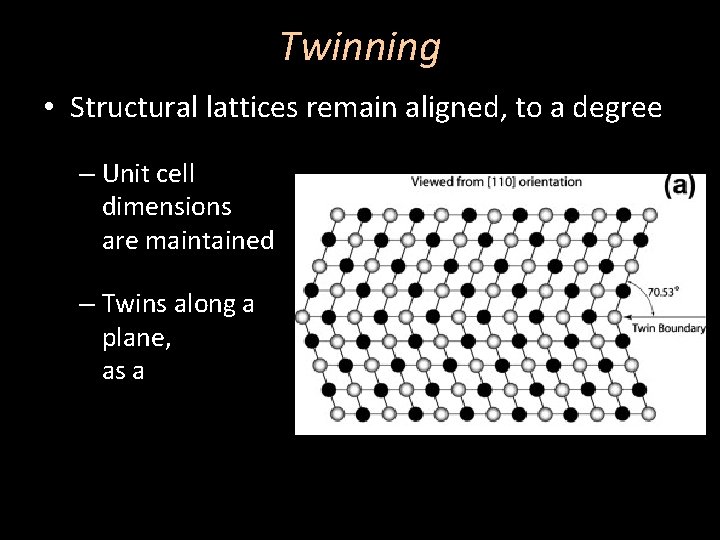
Twinning • Structural lattices remain aligned, to a degree – Unit cell dimensions are maintained – Twins along a plane, as a mirror referred to twin plane
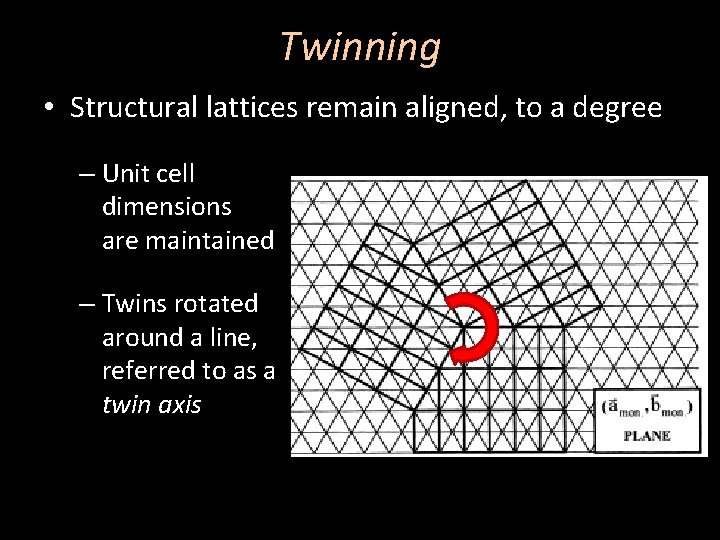
Twinning • Structural lattices remain aligned, to a degree – Unit cell dimensions are maintained – Twins rotated around a line, referred to as a twin axis
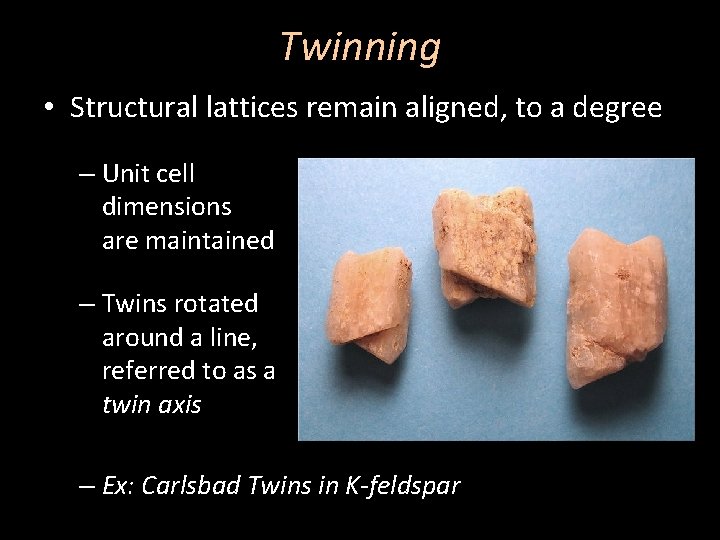
Twinning • Structural lattices remain aligned, to a degree – Unit cell dimensions are maintained – Twins rotated around a line, referred to as a twin axis – Ex: Carlsbad Twins in K-feldspar
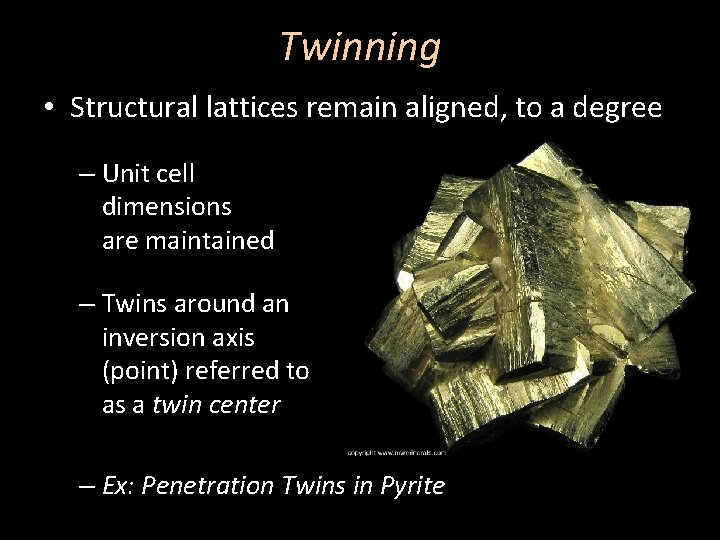
Twinning • Structural lattices remain aligned, to a degree – Unit cell dimensions are maintained – Twins around an inversion axis (point) referred to as a twin center – Ex: Penetration Twins in Pyrite

Twinning • Formed by three fundamental mechanisms – Growth Twins – Transformation Twins – Gliding Twins
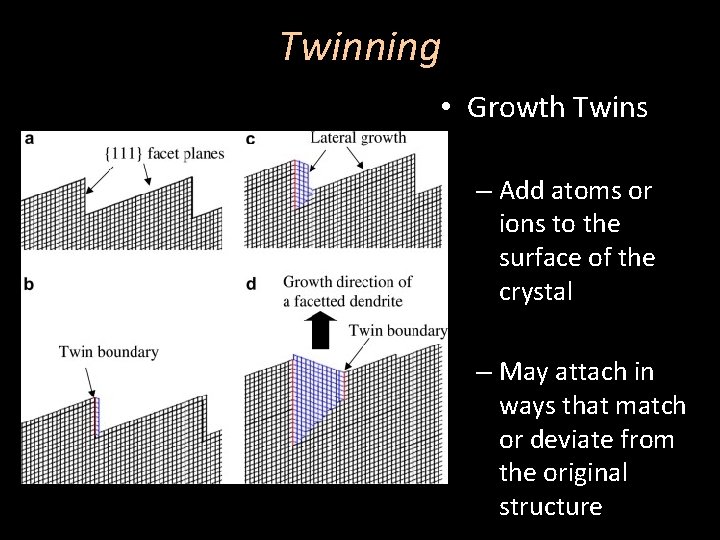
Twinning • Growth Twins – Add atoms or ions to the surface of the crystal – May attach in ways that match or deviate from the original structure

Twinning • Growth Twins – An error initiated during free growth, typically shortly after nucleation – Also referred to as primary twinning
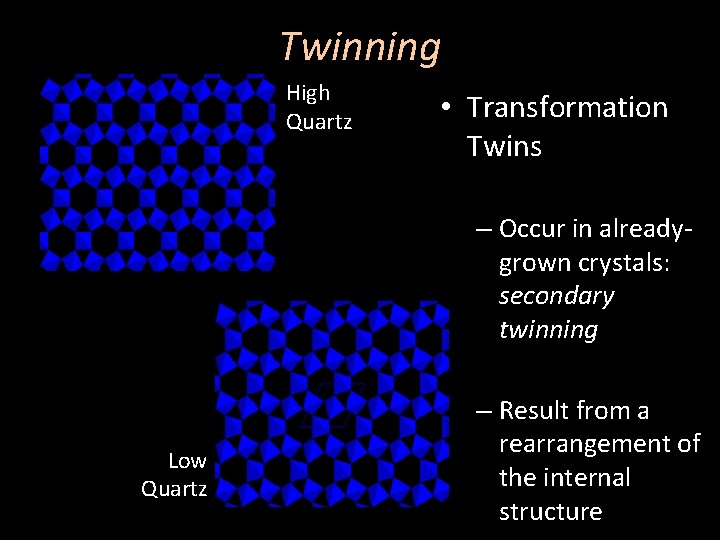
Twinning High Quartz • Transformation Twins – Occur in alreadygrown crystals: secondary twinning Low Quartz – Result from a rearrangement of the internal structure
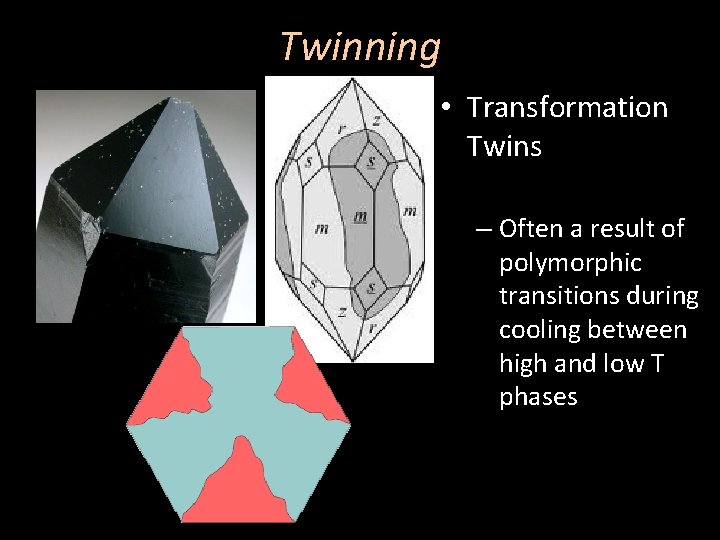
Twinning • Transformation Twins – Often a result of polymorphic transitions during cooling between high and low T phases
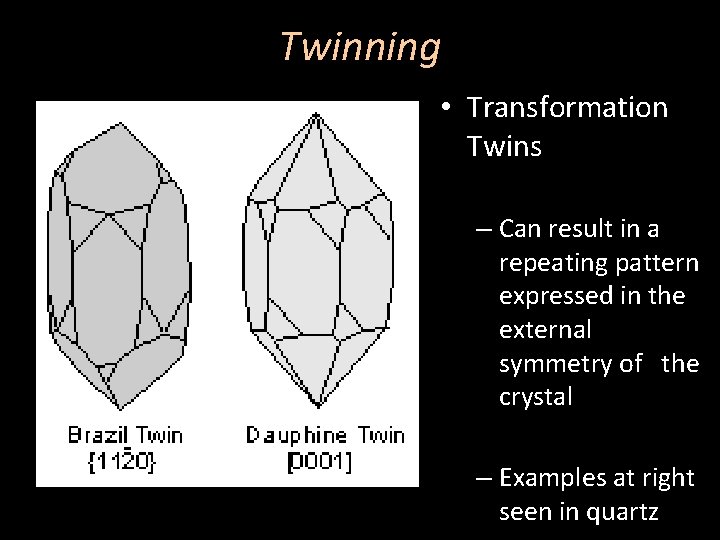
Twinning • Transformation Twins – Can result in a repeating pattern expressed in the external symmetry of the crystal – Examples at right seen in quartz
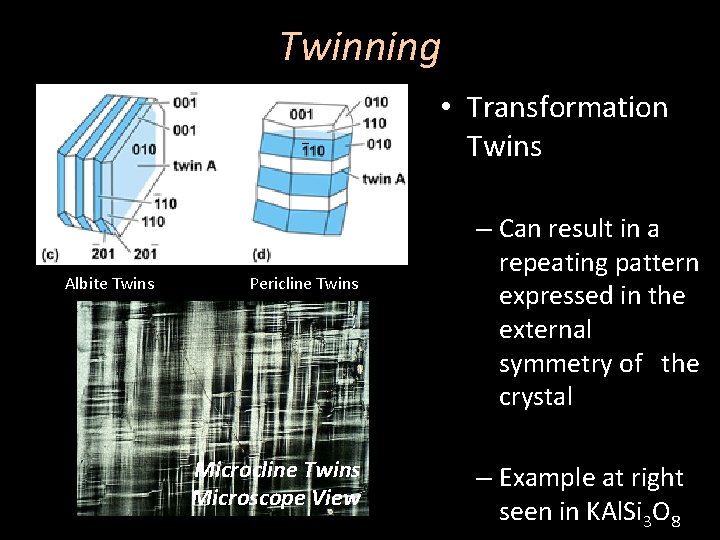
Twinning • Transformation Twins Albite Twins Pericline Twins Microscope View – Can result in a repeating pattern expressed in the external symmetry of the crystal – Example at right seen in KAl. Si 3 O 8
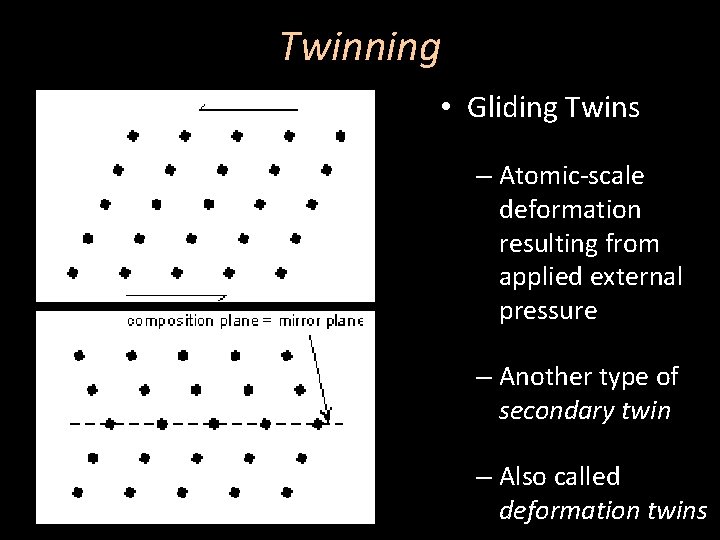
Twinning • Gliding Twins – Atomic-scale deformation resulting from applied external pressure – Another type of secondary twin – Also called deformation twins

Twinning • Gliding Twins – Most-commonly seen in metals – Also seen in metamorphosed calcite and plagioclase crystals as polysynthetic twins
Summary • Mineral Formation – Atoms, ions, or molecules bonding together in a solid, crystalline structure – Three General Processes • Crystallize Solute from Solution • Crystallization of/from Melt • Crystallization from a Vapor
Summary • Initiation of Crystal Growth – Nucleation to bond ions/atoms in an ordered structure – Initially weak attractive force to form additional bonds – Early growth at many sites • Step growth at edges, corners • Clump growth on faces
Summary • Crystal Morphology – Crystal faces most-likely to form parallel to crystal lattice planes • Follow Law of Bravais – Vectorial Properties • Continuous Properties • Discontinuous Properties – Ordered Intergrowth • Parallel Growth • Twinned Growth • Epitaxis

Summary • Structural Defects – Point Defects • Changing points by Substitution, Omission, or Interstitial Methods – Line Defects • Linear Dislocations create defects • Edge and Screw Dislocations – Plane Defects • Lineage Structures, Stacking Faults – Twinning • Symmetric Intergrowths • Twin Planes, Axes, and Centers

Next Time… • Color • Magnetism • Radioactivity
- Slides: 65