Crystal Binding Bonding Continued More on Covalent Bonding
Crystal Binding (Bonding) Continued More on Covalent Bonding Part V

• Consider 2 close Cl atoms. Each has electronic shells 2 2 6 2 5 = 1 s 2 s 2 p 3 s 3 p • If they move close until their outer orbitals overlap, the atoms can share 2 e- & "fill" the remaining 3 p shell of each Cl. • The electronic energy there is lowered, which causes the orbitals to stay overlapped; resulting in a strong bond in Cl 2. This is the covalent or shared electron bond.
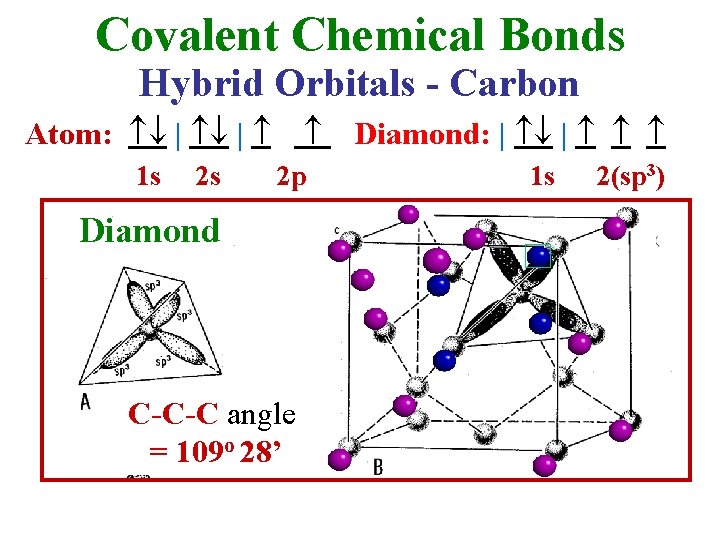
Covalent Chemical Bonds Hybrid Orbitals - Carbon Atom: | | 1 s 2 s 2 p Diamond C-C-C angle = 109 o 28’ Diamond: | | 1 s 2(sp 3)
• The 2(sp 3) orbital is tetrahedrally shaped. Larger Overlap Stronger Bond Covalent Bonds are Directional • Each C is tetrahedrally coordinated with 4 others (& each of them with 4 others. . . ) • The C-C-C bond angle is fixed at 109 o 28' (max. overlap) • Note the Face-Centered Cubic lattice The directional character of the bonds, lower coordination & symmetry, density
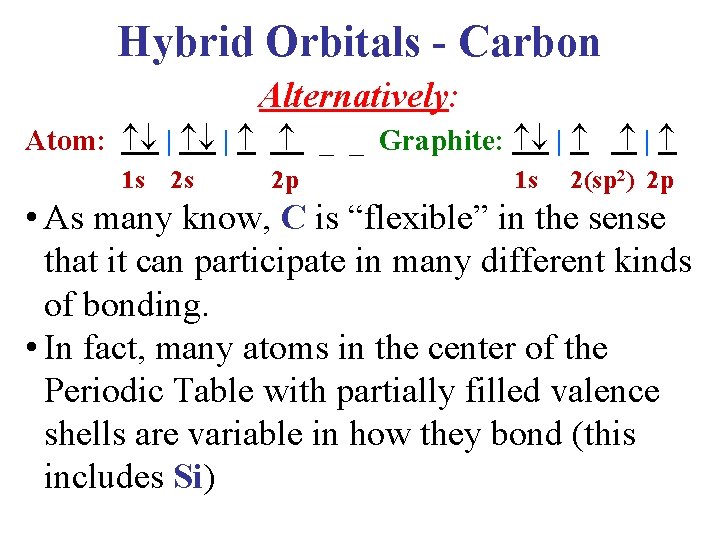
Hybrid Orbitals - Carbon Alternatively: Atom: | | Graphite: | 1 s 2 s 2 p 1 s | 2(sp 2) 2 p • As many know, C is “flexible” in the sense that it can participate in many different kinds of bonding. • In fact, many atoms in the center of the Periodic Table with partially filled valence shells are variable in how they bond (this includes Si)

Covalent Chemical Bonds Graphite Structure

• The 3 2(sp 2) orbitals are coplanar & 120 o apart • The orbital overlap is similar to that in diamond within the planes (strong too!). Belongs to the Hexagonal Crystal Class • Note the p-bonding between the remaining 2 p's • This results in delocalized e- 's in 2 p orbitals which results in electrical conductivity only within sheets. • There are other hybrids as well (dsp 2 in Cu. Oplanar X) e- may resonate in bonds of nonidentical atoms & give a partial ionic character if one much more e-neg than other
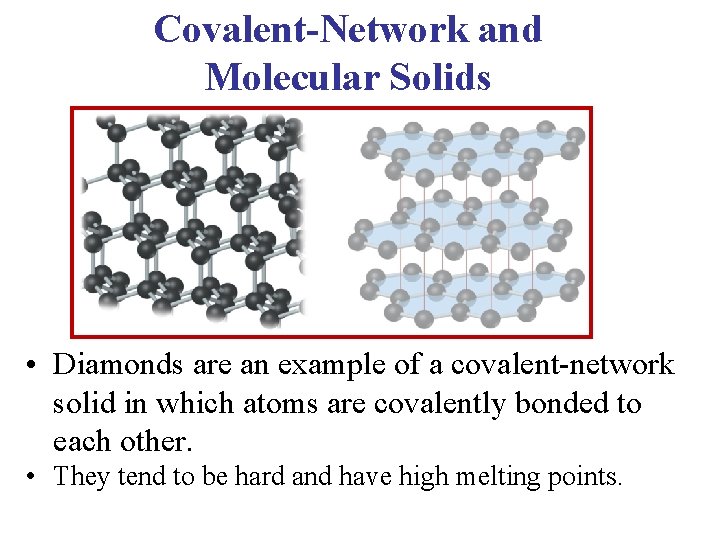
Covalent-Network and Molecular Solids • Diamonds are an example of a covalent-network solid in which atoms are covalently bonded to each other. • They tend to be hard and have high melting points.
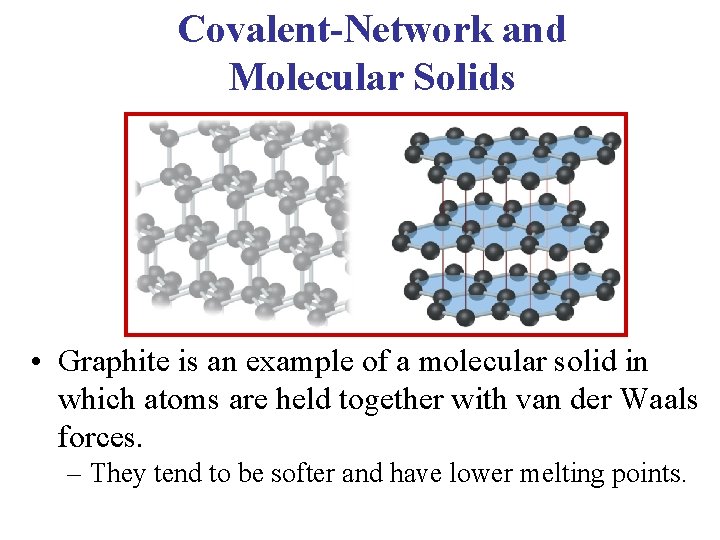
Covalent-Network and Molecular Solids • Graphite is an example of a molecular solid in which atoms are held together with van der Waals forces. – They tend to be softer and have lower melting points.
• Covalent Bonds occur between atoms that are “sharing” electrons: • Form covalent compounds. There is a “tug of war” for the electrons. There can be single, double & triple covalent bonds: • Single bond – a bond in which 2 atoms share a pair of electrons. Double bond – bond that involves 2 shared pairs of e-. Triple bond – bond that involves 3 shared pairs of e-
• Combinations of atoms of non-metallic atoms are likely to form covalent bonds • Groups 4 A, 5 A, 6 A, and 7 A • Summarized by G. Lewis in the octet rule sharing of e- occurs if atoms achieve noble gas configuration, • H 2 is an exception to this rule

Column (Group) Trends • Halogens form single covalent bonds in their diatomic molecules (ex: F – F) • Chalcogens form double covalent bonds in their diatomic molecules (ex: O = O) • Phicogens form triple covalent bonds in their diatomic molecules ( N = N ) • The Carbon group tends to form 4 bonds with other atoms
• As we just briefly saw for C, covalent bonding can be explained using electron configurations and orbital boxes • Double and triple covalent bonds – Oxygen forms a double bond in a diatomic molecule • It is an exception to the octet rule, 2 unpaired e-. – Nitrogen forms a triple bond in a diatomic molecule • Satisfies the octet rule, all e- are paired • Multiple covalent bonds can form between unlike atoms (ex: CO 2, CH 3 OH)
Molecular Orbitals • As we briefly showed, when 2 atoms covalently bond, their atomic orbitals overlap to produce molecular orbitals (orbitals that apply to the entire molecule) • The molecular orbital model of bonding requires that the number of molecular orbitals equal the number of overlapping atomic orbitals • When 2 atomic orbitals overlap, 2 molecular orbitals are created • One is called a bonding orbital, the other is called an anti-bonding orbital • The anti-bonding orbital has a higher energy that the atomic orbitals from which it formed
Molecular Orbitals • When H 2 forms, the 1 s atomic orbitals overlap • 2 electrons are available for bonding (see next slide) • The energy of the e- in the bonding molecular orbital is lower than the e- in the atomic orbitals of the separate H atoms • Electrons seek the lowest energy level, so they fill the bonding molecular orbital • This makes a stable covalent bond between the H atoms • The anti-bonding orbital is empty • Sigma and pi bonds are caused by the overlapping of “s” and “p” orbitals
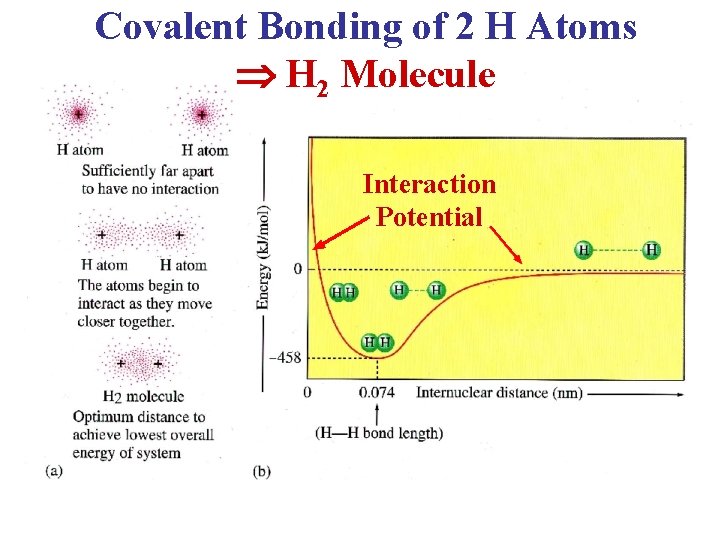
Covalent Bonding of 2 H Atoms H 2 Molecule Interaction Potential

Hybrid Orbitals • In orbital hybridization, several atomic orbitals mix to form the same total number of equivalent hybrid orbitals • One 2 s and three 2 p orbitals of a carbon atom overlap to form an sp 3 hybrid orbital • These are at the tetrahedral angle of 109. 5 o • Four sp 3 orbitals of carbon overlap with the 1 s orbitals of the four hydrogen atoms • This allows for a great deal of overlap, which results in the formation of 4 C-H sigma bonds • These are unusually strong covalent bonds
Bond Polarity • Covalent bonds involve the sharing of electrons • However, they can differ in how the bonds are shared • Depends on the kind and number of atoms joined together When electrons are shared equally, a nonpolar covalent bond is formed • When the atoms share the electron unequally, a polar covalent bond is formed The more electronegative element will have the stronger electron attraction and will acquire a slightly negative charge • The less electronegative element will acquire a slightly positive charge
- Slides: 18