Cryptococcal Antigen Cr Ag Essential In Vitro Diagnostic






- Slides: 24

Cryptococcal Antigen (Cr. Ag) Essential In Vitro Diagnostic Device David R Boulware MD, MPH, CTrop. Med Associate Professor University of Minnesota, USA boulw 001@umn. edu

Overview • • Need Existing Cr. Ag LFAs Existing Validation Data Discussion

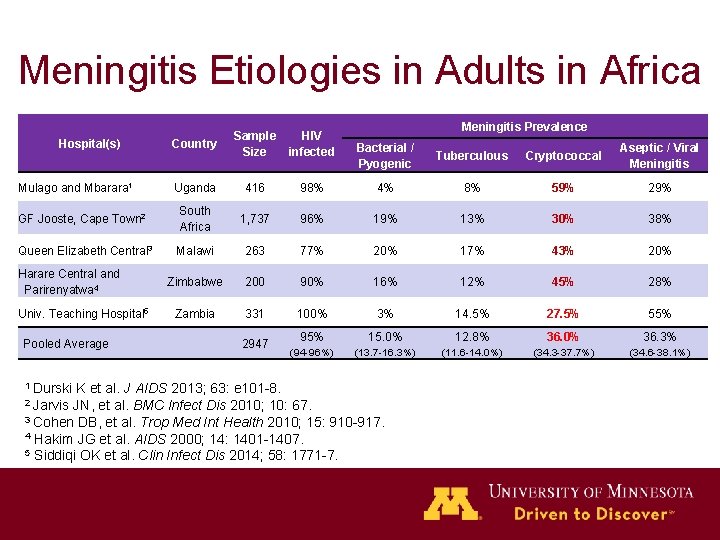
Meningitis Etiologies in Adults in Africa Country Sample Size HIV infected Uganda 416 GF Jooste, Cape Town 2 South Africa Queen Elizabeth Central 3 Hospital(s) Mulago and Mbarara 1 Harare Central and Parirenyatwa 4 Univ. Teaching Hospital 5 Pooled Average 1 Durski K et al. J Meningitis Prevalence Bacterial / Pyogenic Tuberculous Cryptococcal Aseptic / Viral Meningitis 98% 4% 8% 59% 29% 1, 737 96% 19% 13% 30% 38% Malawi 263 77% 20% 17% 43% 20% Zimbabwe 200 90% 16% 12% 45% 28% Zambia 331 100% 3% 14. 5% 27. 5% 55% 2947 95% 15. 0% 12. 8% 36. 0% 36. 3% (94 -96%) (13. 7 -16. 3%) (11. 6 -14. 0%) (34. 3 -37. 7%) (34. 6 -38. 1%) AIDS 2013; 63: e 101 -8. Infect Dis 2010; 10: 67. 3 Cohen DB, et al. Trop Med Int Health 2010; 15: 910 -917. 4 Hakim JG et al. AIDS 2000; 14: 1401 -1407. 5 Siddiqi OK et al. Clin Infect Dis 2014; 58: 1771 -7. 2 Jarvis JN, et al. BMC

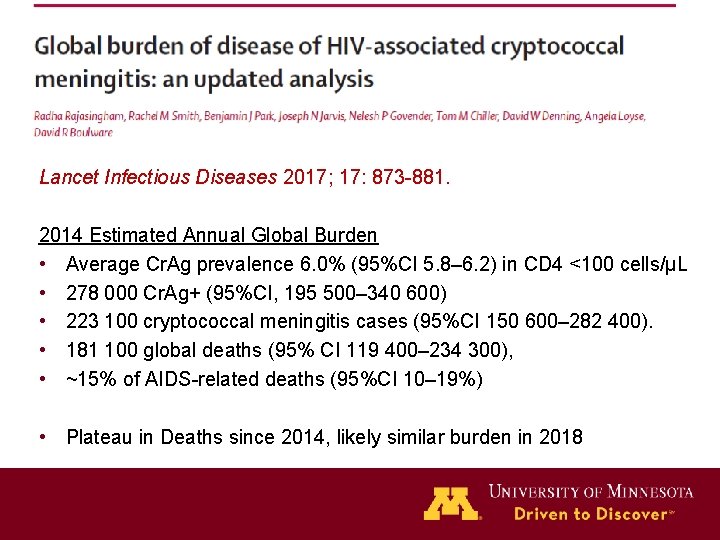
Lancet Infectious Diseases 2017; 17: 873 -881. 2014 Estimated Annual Global Burden • Average Cr. Ag prevalence 6. 0% (95%CI 5. 8– 6. 2) in CD 4 <100 cells/μL • 278 000 Cr. Ag+ (95%CI, 195 500– 340 600) • 223 100 cryptococcal meningitis cases (95%CI 150 600– 282 400). • 181 100 global deaths (95% CI 119 400– 234 300), • ~15% of AIDS-related deaths (95%CI 10– 19%) • Plateau in Deaths since 2014, likely similar burden in 2018

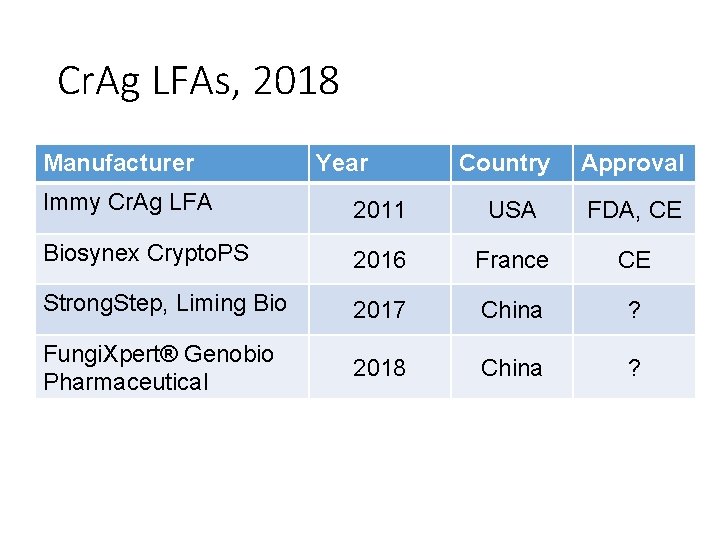
Cr. Ag LFAs, 2018 Manufacturer Year Country Approval Immy Cr. Ag LFA 2011 USA FDA, CE Biosynex Crypto. PS 2016 France CE Strong. Step, Liming Bio 2017 China ? Fungi. Xpert® Genobio Pharmaceutical 2018 China ?

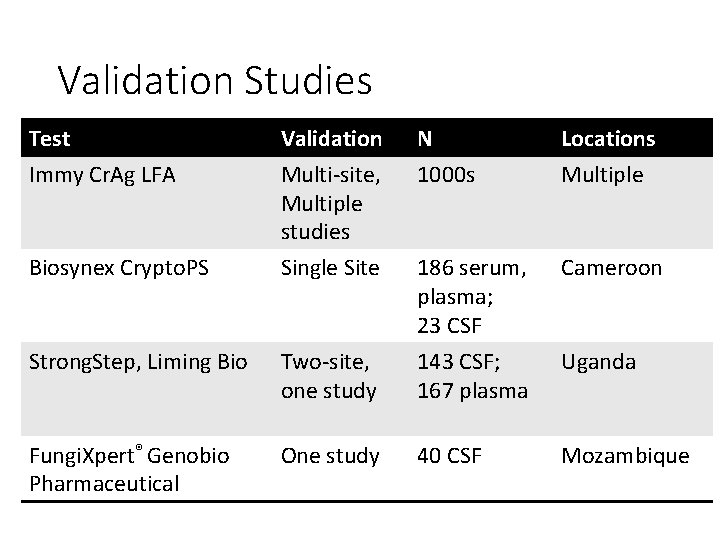
Validation Studies Test Immy Cr. Ag LFA Validation Multi-site, Multiple studies N 1000 s Locations Multiple Biosynex Crypto. PS Single Site Cameroon Strong. Step, Liming Bio Two-site, one study 186 serum, plasma; 23 CSF 143 CSF; 167 plasma Fungi. Xpert® Genobio Pharmaceutical One study 40 CSF Mozambique Uganda

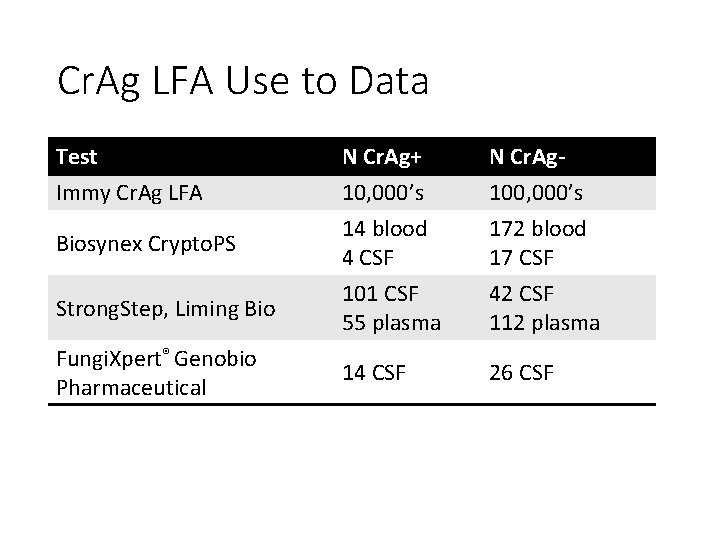
Cr. Ag LFA Use to Data Test Immy Cr. Ag LFA N Cr. Ag+ 10, 000’s 14 blood 4 CSF N Cr. Ag 100, 000’s 172 blood 17 CSF Strong. Step, Liming Bio 101 CSF 55 plasma 42 CSF 112 plasma Fungi. Xpert® Genobio Pharmaceutical 14 CSF 26 CSF Biosynex Crypto. PS

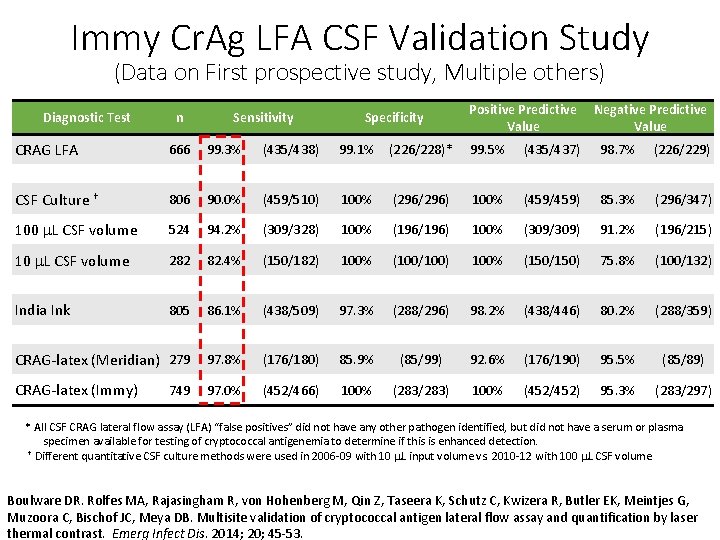
Immy Cr. Ag LFA CSF Validation Study (Data on First prospective study, Multiple others) Diagnostic Test CRAG LFA n Sensitivity Specificity Positive Predictive Value Negative Predictive Value 666 99. 3% (435/438) 99. 1% (226/228)* 99. 5% (435/437) 98. 7% (226/229) CSF Culture † 806 90. 0% (459/510) 100% (296/296) 100% (459/459) 85. 3% (296/347) 100 m. L CSF volume 524 94. 2% (309/328) 100% (196/196) 100% (309/309) 91. 2% (196/215) 10 m. L CSF volume 282 82. 4% (150/182) 100% (100/100) 100% (150/150) 75. 8% (100/132) 805 86. 1% (438/509) 97. 3% (288/296) 98. 2% (438/446) 80. 2% (288/359) CRAG-latex (Meridian) 279 97. 8% (176/180) 85. 9% (85/99) 92. 6% (176/190) 95. 5% (85/89) CRAG-latex (Immy) 97. 0% (452/466) 100% (283/283) 100% (452/452) 95. 3% (283/297) India Ink 749 * All CSF CRAG lateral flow assay (LFA) “false positives” did not have any other pathogen identified, but did not have a serum or plasma specimen available for testing of cryptococcal antigenemia to determine if this is enhanced detection. † Different quantitative CSF culture methods were used in 2006 -09 with 10 m. L input volume vs. 2010 -12 with 100 m. L CSF volume Boulware DR. Rolfes MA, Rajasingham R, von Hohenberg M, Qin Z, Taseera K, Schutz C, Kwizera R, Butler EK, Meintjes G, Muzoora C, Bischof JC, Meya DB. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014; 20; 45 -53.

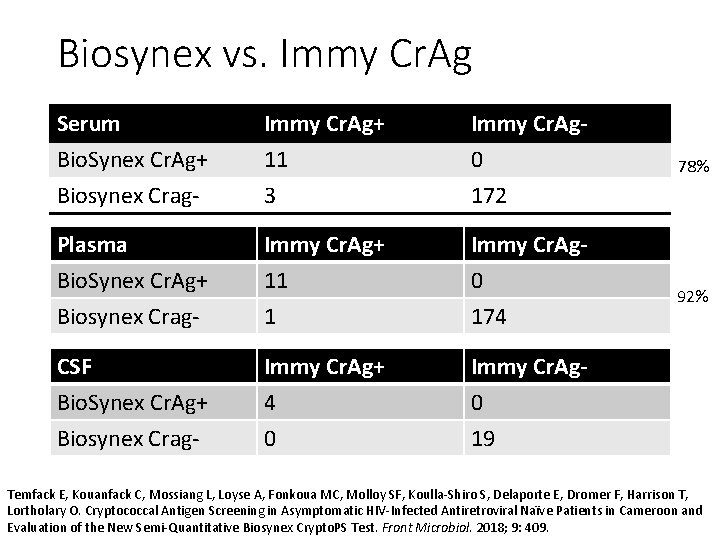
Biosynex vs. Immy Cr. Ag Serum Bio. Synex Cr. Ag+ Biosynex Crag- Immy Cr. Ag+ 11 3 Immy Cr. Ag 0 172 Plasma Bio. Synex Cr. Ag+ Biosynex Crag- Immy Cr. Ag+ 11 1 Immy Cr. Ag 0 174 CSF Bio. Synex Cr. Ag+ Biosynex Crag- Immy Cr. Ag+ 4 0 Immy Cr. Ag 0 19 78% 92% Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla-Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. Cryptococcal Antigen Screening in Asymptomatic HIV-Infected Antiretroviral Naïve Patients in Cameroon and Evaluation of the New Semi-Quantitative Biosynex Crypto. PS Test. Front Microbiol. 2018; 9: 409.

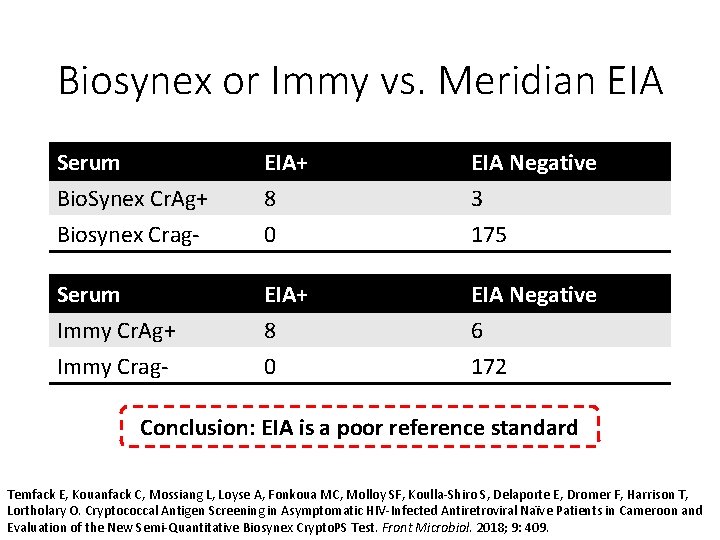
Biosynex or Immy vs. Meridian EIA Serum Bio. Synex Cr. Ag+ Biosynex Crag- EIA+ 8 0 EIA Negative 3 175 Serum Immy Cr. Ag+ Immy Crag- EIA+ 8 0 EIA Negative 6 172 Conclusion: EIA is a poor reference standard Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla-Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. Cryptococcal Antigen Screening in Asymptomatic HIV-Infected Antiretroviral Naïve Patients in Cameroon and Evaluation of the New Semi-Quantitative Biosynex Crypto. PS Test. Front Microbiol. 2018; 9: 409.

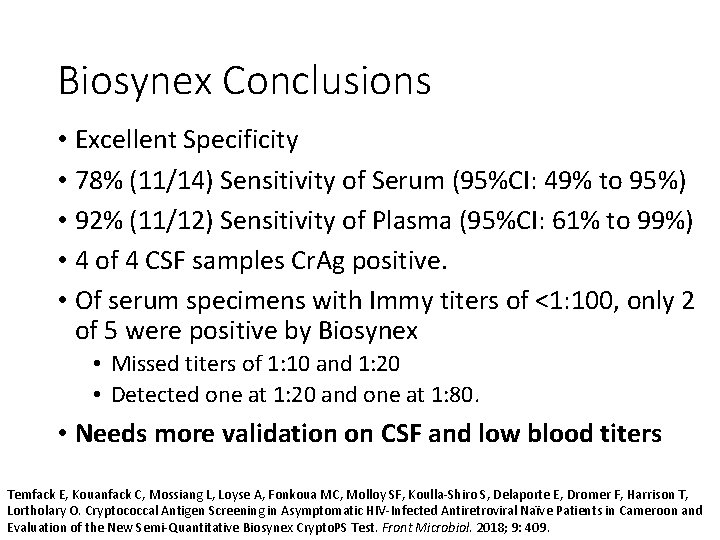
Biosynex Conclusions • Excellent Specificity • 78% (11/14) Sensitivity of Serum (95%CI: 49% to 95%) • 92% (11/12) Sensitivity of Plasma (95%CI: 61% to 99%) • 4 of 4 CSF samples Cr. Ag positive. • Of serum specimens with Immy titers of <1: 100, only 2 of 5 were positive by Biosynex • Missed titers of 1: 10 and 1: 20 • Detected one at 1: 20 and one at 1: 80. • Needs more validation on CSF and low blood titers Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla-Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. Cryptococcal Antigen Screening in Asymptomatic HIV-Infected Antiretroviral Naïve Patients in Cameroon and Evaluation of the New Semi-Quantitative Biosynex Crypto. PS Test. Front Microbiol. 2018; 9: 409.

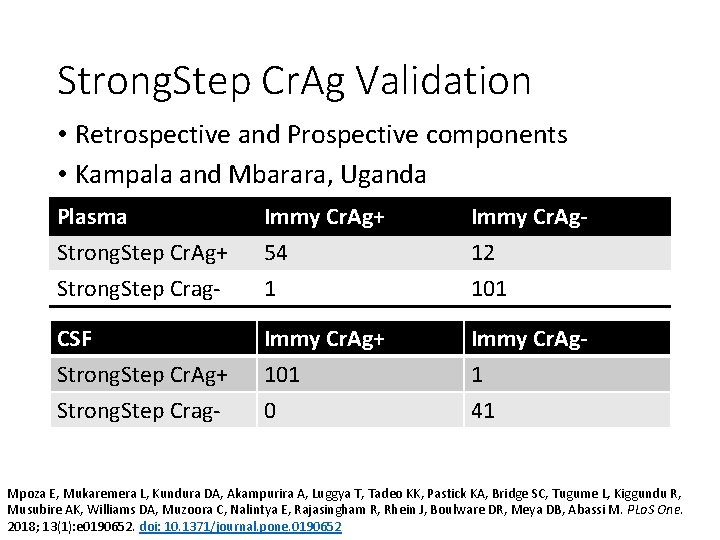
Strong. Step Cr. Ag Validation • Retrospective and Prospective components • Kampala and Mbarara, Uganda Plasma Strong. Step Cr. Ag+ Strong. Step Crag- Immy Cr. Ag+ 54 1 Immy Cr. Ag 12 101 CSF Strong. Step Cr. Ag+ Strong. Step Crag- Immy Cr. Ag+ 101 0 Immy Cr. Ag 1 41 Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. PLo. S One. 2018; 13(1): e 0190652. doi: 10. 1371/journal. pone. 0190652

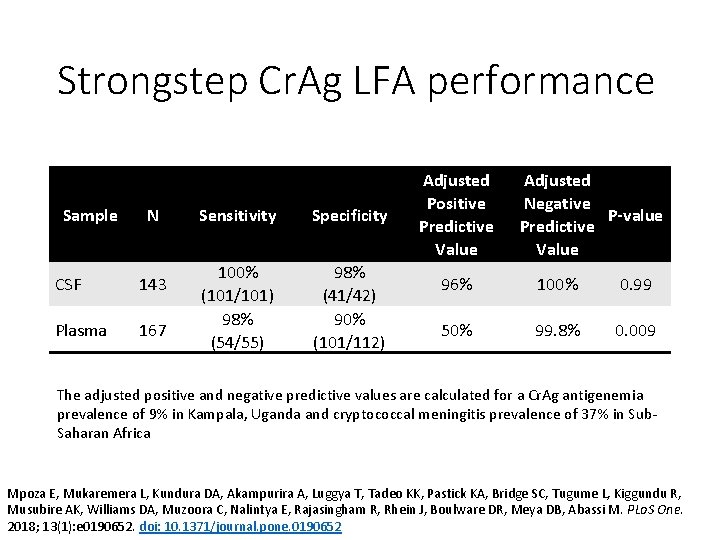
Strongstep Cr. Ag LFA performance Sample N CSF 143 Plasma 167 Sensitivity Specificity 100% (101/101) 98% (54/55) 98% (41/42) 90% (101/112) Adjusted Positive Predictive Value Adjusted Negative P-value Predictive Value 96% 100% 0. 99 50% 99. 8% 0. 009 The adjusted positive and negative predictive values are calculated for a Cr. Ag antigenemia prevalence of 9% in Kampala, Uganda and cryptococcal meningitis prevalence of 37% in Sub. Saharan Africa Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. PLo. S One. 2018; 13(1): e 0190652. doi: 10. 1371/journal. pone. 0190652

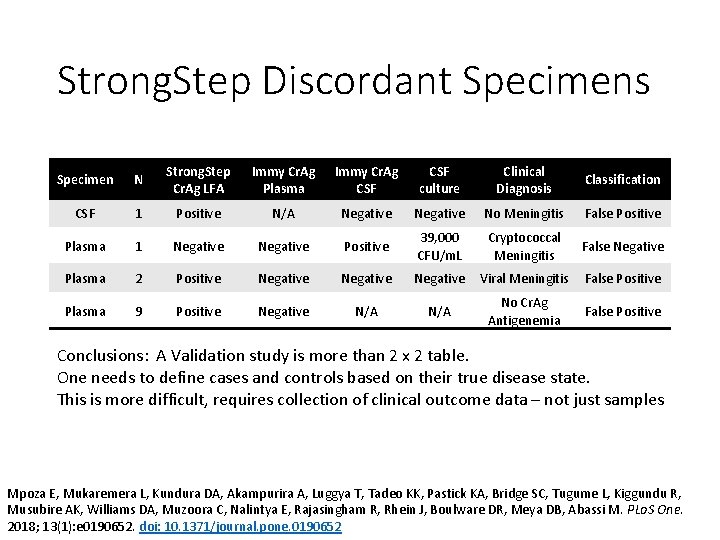
Strong. Step Discordant Specimens Specimen N Strong. Step Cr. Ag LFA Immy Cr. Ag Plasma Immy Cr. Ag CSF culture Clinical Diagnosis Classification CSF 1 Positive N/A Negative No Meningitis False Positive Plasma 1 Negative Positive 39, 000 CFU/m. L Cryptococcal Meningitis False Negative Plasma 2 Positive Negative Viral Meningitis False Positive Plasma 9 Positive Negative N/A No Cr. Ag Antigenemia False Positive Conclusions: A Validation study is more than 2 x 2 table. One needs to define cases and controls based on their true disease state. This is more difficult, requires collection of clinical outcome data – not just samples Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. PLo. S One. 2018; 13(1): e 0190652. doi: 10. 1371/journal. pone. 0190652

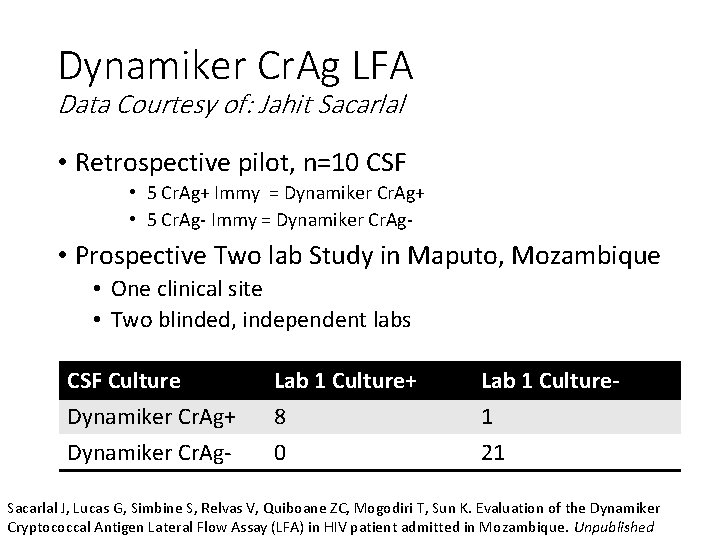
Dynamiker Cr. Ag LFA Data Courtesy of: Jahit Sacarlal • Retrospective pilot, n=10 CSF • 5 Cr. Ag+ Immy = Dynamiker Cr. Ag+ • 5 Cr. Ag- Immy = Dynamiker Cr. Ag- • Prospective Two lab Study in Maputo, Mozambique • One clinical site • Two blinded, independent labs CSF Culture Dynamiker Cr. Ag+ Dynamiker Cr. Ag- Lab 1 Culture+ 8 0 Lab 1 Culture 1 21 Sacarlal J, Lucas G, Simbine S, Relvas V, Quiboane ZC, Mogodiri T, Sun K. Evaluation of the Dynamiker Cryptococcal Antigen Lateral Flow Assay (LFA) in HIV patient admitted in Mozambique. Unpublished

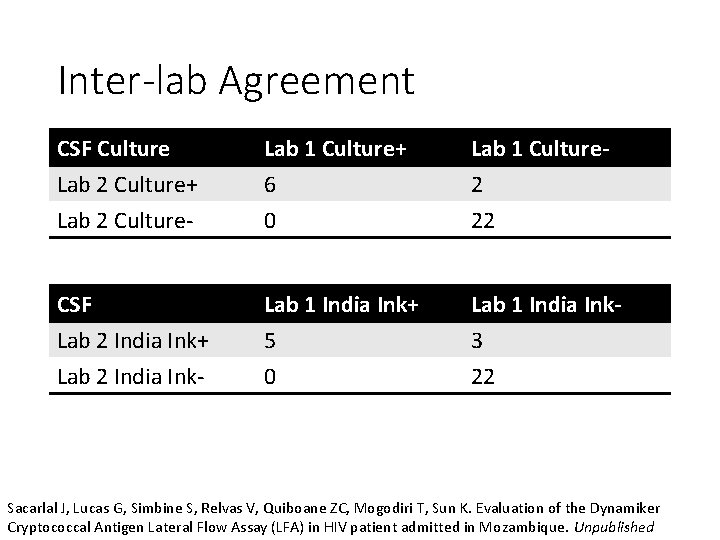
Inter-lab Agreement CSF Culture Lab 2 Culture+ Lab 2 Culture- Lab 1 Culture+ 6 0 Lab 1 Culture 2 22 CSF Lab 2 India Ink+ Lab 2 India Ink- Lab 1 India Ink+ 5 0 Lab 1 India Ink 3 22 Sacarlal J, Lucas G, Simbine S, Relvas V, Quiboane ZC, Mogodiri T, Sun K. Evaluation of the Dynamiker Cryptococcal Antigen Lateral Flow Assay (LFA) in HIV patient admitted in Mozambique. Unpublished

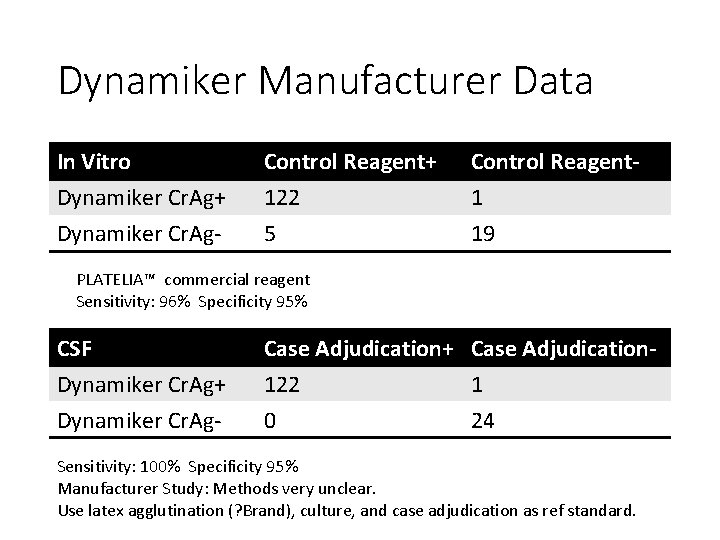
Dynamiker Manufacturer Data In Vitro Dynamiker Cr. Ag+ Dynamiker Cr. Ag- Control Reagent+ 122 5 Control Reagent 1 19 PLATELIA™ commercial reagent Sensitivity: 96% Specificity 95% CSF Dynamiker Cr. Ag+ Dynamiker Cr. Ag- Case Adjudication+ Case Adjudication 122 1 0 24 Sensitivity: 100% Specificity 95% Manufacturer Study: Methods very unclear. Use latex agglutination (? Brand), culture, and case adjudication as ref standard.

References Strong. Step • Mpoza E, Mukaremera L, Kundura DA, Akampurira A, Luggya T, Tadeo KK, Pastick KA, Bridge SC, Tugume L, Kiggundu R, Musubire AK, Williams DA, Muzoora C, Nalintya E, Rajasingham R, Rhein J, Boulware DR, Meya DB, Abassi M. PLo. S One. 2018; 13(1): e 0190652. doi: 10. 1371/journal. pone. 0190652 Biosynex • Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, Koulla. Shiro S, Delaporte E, Dromer F, Harrison T, Lortholary O. Cryptococcal Antigen Screening in Asymptomatic HIV-Infected Antiretroviral Naïve Patients in Cameroon and Evaluation of the New Semi-Quantitative Biosynex Crypto. PS Test. Front Microbiol. 2018 Mar 13; 9: 409. doi: 10. 3389/fmicb. 2018. 00409. Dynamiker • Sacarlal J, Lucas G, Simbine S, Relvas V, Quiboane ZC, Mogodiri T, Sun K. Evaluation of the Dynamiker Cryptococcal Antigen Lateral Flow Assay (LFA) in HIV patient admitted in Mozambique. Unpublished

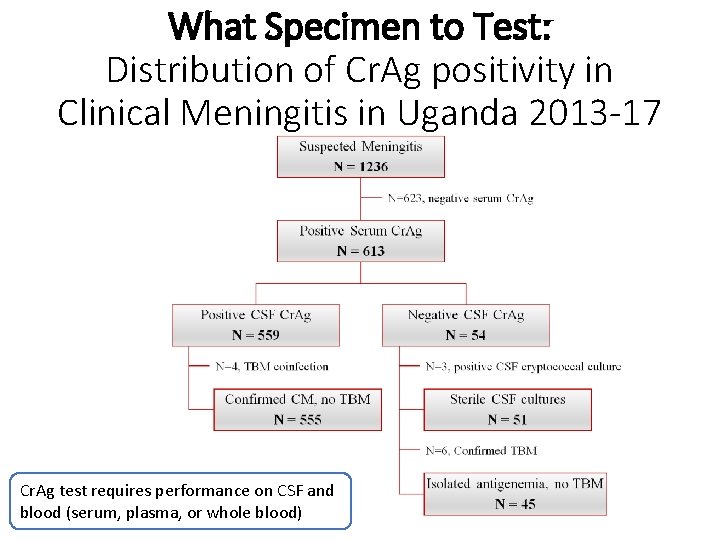
What Specimen to Test: Distribution of Cr. Ag positivity in Clinical Meningitis in Uganda 2013 -17 Cr. Ag test requires performance on CSF and blood (serum, plasma, or whole blood)

Discussion • General Discussion • Information to be included with an application for inclusion in the WHO List of Essential In Vitro Diagnostics Device (IVD)s.

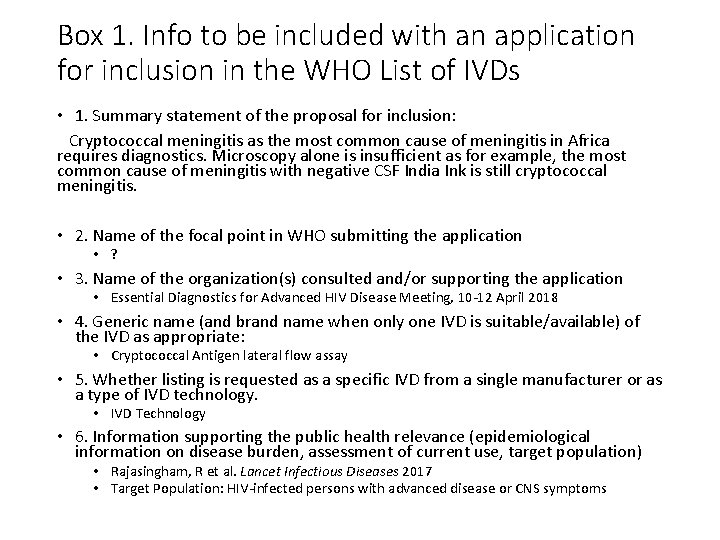
Box 1. Info to be included with an application for inclusion in the WHO List of IVDs • 1. Summary statement of the proposal for inclusion: Cryptococcal meningitis as the most common cause of meningitis in Africa requires diagnostics. Microscopy alone is insufficient as for example, the most common cause of meningitis with negative CSF India Ink is still cryptococcal meningitis. • 2. Name of the focal point in WHO submitting the application • ? • 3. Name of the organization(s) consulted and/or supporting the application • Essential Diagnostics for Advanced HIV Disease Meeting, 10 -12 April 2018 • 4. Generic name (and brand name when only one IVD is suitable/available) of the IVD as appropriate: • Cryptococcal Antigen lateral flow assay • 5. Whether listing is requested as a specific IVD from a single manufacturer or as a type of IVD technology. • IVD Technology • 6. Information supporting the public health relevance (epidemiological information on disease burden, assessment of current use, target population) • Rajasingham, R et al. Lancet Infectious Diseases 2017 • Target Population: HIV-infected persons with advanced disease or CNS symptoms

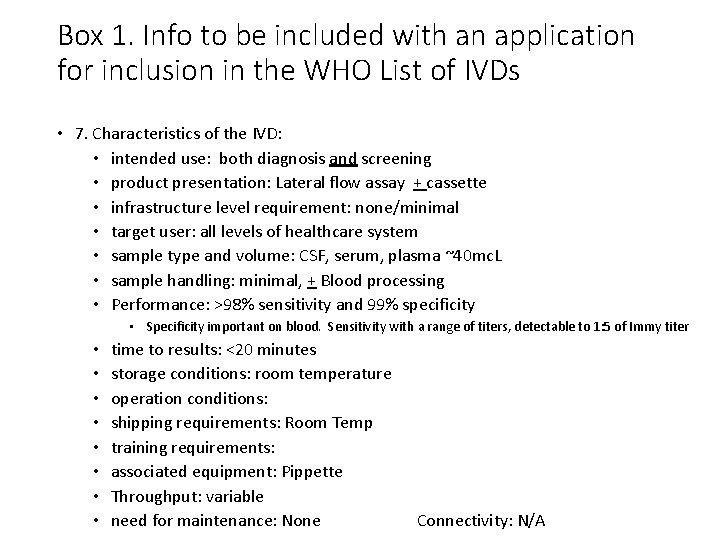
Box 1. Info to be included with an application for inclusion in the WHO List of IVDs • 7. Characteristics of the IVD: • intended use: both diagnosis and screening • product presentation: Lateral flow assay + cassette • infrastructure level requirement: none/minimal • target user: all levels of healthcare system • sample type and volume: CSF, serum, plasma ~40 mc. L • sample handling: minimal, + Blood processing • Performance: >98% sensitivity and 99% specificity • Specificity important on blood. Sensitivity with a range of titers, detectable to 1: 5 of Immy titer • • time to results: <20 minutes storage conditions: room temperature operation conditions: shipping requirements: Room Temp training requirements: associated equipment: Pippette Throughput: variable need for maintenance: None Connectivity: N/A

Box 1. Info to be included with an application for inclusion in the WHO List of IVDs • 8. Use of the IVD details (as part of a diagnostic testing algorithm; reference to existing WHO and other clinical guidelines; need for special diagnostic or treatment facilities and skills) • Pre-ART Screening in CD 4<200 as per 2017 WHO Guidelines for Advanced HIV Disease • Suspected Meningitis (Blood and CSF) • 9. Summary of laboratory evaluation studies • Previously Summarized (Expand for prior published Immy studies) • 10. Summary of validation studies in a variety of clinical settings: • Identification of clinical evidence (search strategy, systematic reviews identified, reasons for selection/exclusion of particular data) • Summary of available data (appraisal of quality, performance, ease of use, summary of results)

Box 1. Info to be included with an application for inclusion in the WHO List of IVDs 11. Summary of evidence on safety. N/A 12. Summary of data on comparative cost and cost-effectiveness: • Screening: Rajasingham JAIDS 2012; Jarvis PLo. S One 2013 • Meningitis Dx: Durski K. JAIDS 2013 13. Summary of regulatory status of the IVD: • FDA Approval: Immy • CE Mark (EU): Immy, Biosynex • WHO PQ: Not available from WHO for most common cause of meningitis. 14. Proposed text for the WHO List of Essential IVDs. • Text: