Cryopreservation of fish gametes What is cryopreservation Cryopreservation












- Slides: 15

Cryopreservation of fish gametes

What is cryopreservation? • Cryopreservation is the long term preservation of biological material at ultra-low temperatures, usually at -196 0 C, the temperature of liquid nitrogen. • At this temperature, the cellular viability can be stored in a genetically stable form and is affected only by background radiation.

Why cryopreservation? Cryopreservation has several practical applications in fisheries and aquaculture. They are : 1. Wider distribution of gametes from one location to another location 2. Reduces number of male broodfish to be maintained 3. Facilitates extension of period of seed availability 4. Selective breeding programmes wherein a large number of families have to be maintained 5. Production of androgenetic fish 6. Conservation of genetic resources

Cryopreservation of fish spermatozoa: • The spermatozoa of several species of finfish and shellfish have been cryopreserved and `Sperm banks’ established for some species. This is because of : -Smaller size (4 -6 um) -Larger number per unit volume (several million spermatozoa/ml milt) -Repeatability and ease of collection and handling -Simple membrane (easy to dehydrate or cryoprotect spermatozoa) Figure: Minute spermatozoa found within seminiferous lobule Figure: A mature egg (oocyte)

Handling of spermatozoa prior to freezing • Collection of spermatozoa from mature male, avoiding contamination with urine, mucus, water, faeces, etc. Collection of spermatozoa • Males may be injected with spawning agent to ensure higher milt volume • Motility test to be carried out to ensure milt quality • Spermatozoa showing 70% or more motility should be selected for cryopreservation

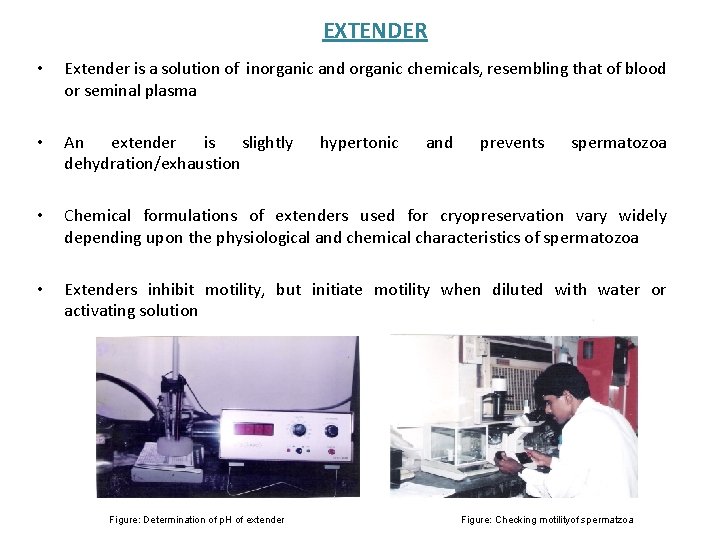
EXTENDER • Extender is a solution of inorganic and organic chemicals, resembling that of blood or seminal plasma • An extender is slightly dehydration/exhaustion • Chemical formulations of extenders used for cryopreservation vary widely depending upon the physiological and chemical characteristics of spermatozoa • Extenders inhibit motility, but initiate motility when diluted with water or activating solution Figure: Determination of p. H of extender hypertonic and prevents spermatozoa Figure: Checking motilityof spermatzoa

CRYOPROTECTANT : • Cryoprotectant is added to extender – milt mixture to minimize freeze-damage to cells during cooling/ freezing. • Common cryoprotectants recommended are – dimethyl sulfoxide (DMSO), methanol, glycerol, DMA, etc. • The optimum concentration of cryoprotectant is 5 -15% of the total volume of the diluent (extender + milt + cryoprotectant). • Cryoprotectants are of two types- intracellular (penetrating) and extracellular (non -penetrating) • Equilibration period : It is the time allowed for cryoprotectant to penetrate into the sperm cells. It may vary from a few minutes to several minutes.

Storage containers: • Diluted spermatozoa are normally stored in polypropylene vials (1 -2 ml), as pellets (40 -200 µl) and in 0. 25 or 0. 50 ml plastic and then it forms a seal when comes in contact with a fluid. Figure: Filling plastic straw with diluted spermatozoa

Dilution ratio: • The spz : diluent ratio varies between 1: 1 and 1: 10, depending upon species. • The dilution ratio should be such that the spz need not be diluted further at the time of fertilization.

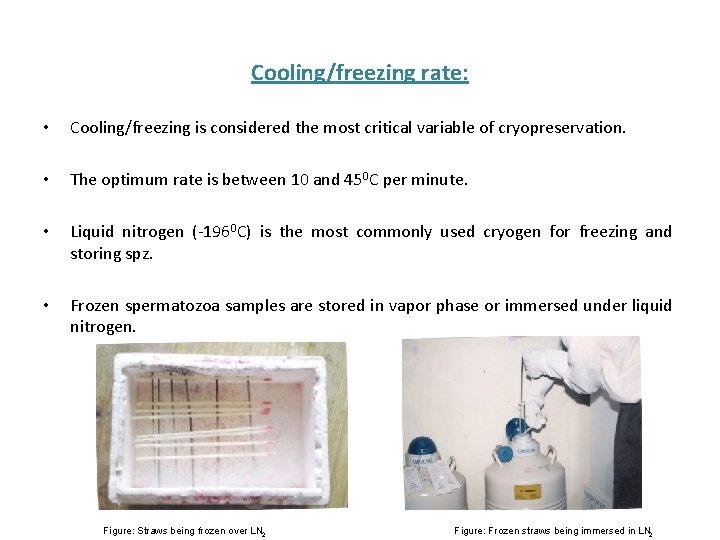
Cooling/freezing rate: • Cooling/freezing is considered the most critical variable of cryopreservation. • The optimum rate is between 10 and 450 C per minute. • Liquid nitrogen (-1960 C) is the most commonly used cryogen for freezing and storing spz. • Frozen spermatozoa samples are stored in vapor phase or immersed under liquid nitrogen. Figure: Straws being frozen over LN 2 Figure: Frozen straws being immersed in LN 2

Warming/thawing rate: • Thawing is also considered an important variable in cryopreservation. • Very rapid thawing rates are used to avoid recrystallization. • Slow warming rate may result in recrystallization. • Thawing of preserved spz is accomplished by agitating them in hot-water bath at 370 C for 10 -15 seconds • Thawing rates of 50 -700 C are recommended, although higher rates of 100 -15000 C have also been used. Figure: Straws being thawed at 370 C

Viability of cryopreserved spermatozoa : • Spermatozoa stored under LN 2 remain fertile indefinite. • They should be thawed only when required for checking motility. • Motility, fertilization and hatching rates, fry survival, etc. are the common criteria for judging the post-thaw viability/fertility of cryopreserved spermatozoa

Cryopreservation of eggs/embryos: • No success • The fundamental problems are – insufficient dehydration during cooling/freezing due to relatively large size (1 -6 mm) of fish eggs, the presence of membranes of different water permeability. • Also permeation of cryoprotectant even into smaller eggs and embryos is low. • However, success has been achieved with invertebrate eggs and embryos. • Sea urchin embryos, oyster larvae (trochophore) and penaied shrimp naupli have been successfully cryopreserved and revived.

Embryonic cell cryopreservation: • Studies show that the cells from blastula can be removed and successfully cryopreserved • This involves dechorionization of unfertilized water-hardened egg, removal of nucleus from the egg, micro-injection of thawed dissociated cells from midblastula into the enucleated egg and its subsequent development into a viable embryo

 Cryopreservation of fish gametes
Cryopreservation of fish gametes One fish two fish red fish blue fish ride
One fish two fish red fish blue fish ride Scale types fish
Scale types fish Twofish and blowfish
Twofish and blowfish Disadvantages of cryopreservation
Disadvantages of cryopreservation Lions eye hospital
Lions eye hospital Genotypic ratio of dihybrid cross
Genotypic ratio of dihybrid cross Gonads
Gonads Two traits punnett square
Two traits punnett square Meiosis makes gametes
Meiosis makes gametes Interesting facts about the integumentary system
Interesting facts about the integumentary system How to do the foil method in biology
How to do the foil method in biology What are gametes
What are gametes Blood type genetics
Blood type genetics Gametes
Gametes Meiosis diagram
Meiosis diagram