Cryogenic Safety What are Cryogenics All cryogenic liquids















- Slides: 20

Cryogenic Safety

What are Cryogenics? All cryogenic liquids are gases at room temperature and atmospheric pressure. The gases are liquefied at very low temperature and high pressures. Different cryogens become liquids under different conditions of temperature and pressures. Cryogenic liquids are materials with boiling points of less than – 73 °C (– 100 °F). •

Cryogen Background • When stored and used properly, cryogens are safe. • If stored improperly or misused, cryogen can be dangerous and even kill you. Gaby Scanlon 10/2012

Two Common Properties Extremely cold in liquid state Small quantities of liquid may expand to large volumes of gas

Which Cryogenics are used in the Physics Department? • Liquid Nitrogen • Liquid Helium • Dry Ice (Carbon Dioxide)

Liquid Nitrogen is heavier than air, inert, colorless, non-corrosive, non-flammable, and extremely cold. • Nitrogen will not react with other elements or compounds under ordinary conditions. • • Boiling Point at 1 atm is -195. 8°C/-320. 4°F/77 K • Expansion Ratio, (liquid to gas): 1 to 694 Under certain conditions, nitrogen can react violently with lithium, neodymium, titanium (above 1472°F/800°C), and magnesium to form nitrides. At high temperature, it can also combine with oxygen and hydrogen. •

Liquid Helium Liquid Nitrogen is lighter than air, inert, colorless, odorless, noncorrosive, non-flammable, and extremely cold. • Helium will not react with other elements or compounds under ordinary conditions. • • Boiling Point at 1 atm is -268. 9°C/-452. 1°F/4 K • Expansion Ratio, (liquid to gas): 1 to 754

Dry Ice (Carbon Dioxide) Carbon Dioxide is typically in a solidified gas state such as nuggets, pellets, or blocks. Dry Ice appears white in color, is non-corrosive, non-flammable, and extremely cold. Vapors are heavier than air. • Carbon Dioxide is incompatible with alkali metals, alkaline earth metals, metal acetylides, chromium, titanium above 1022°F (550°C), uranium above 1382°F (750°C), magnesium above 1427°F (775°C) • • Boiling Point at 1 atm is -78. 5°C/-109. 3°F/195 K • Expansion Ratio, (Solid to gas): 1 to 554 Carbon monoxide and oxygen may result from the decomposition of carbon dioxide exposed to electrical discharges and high temperatures. •

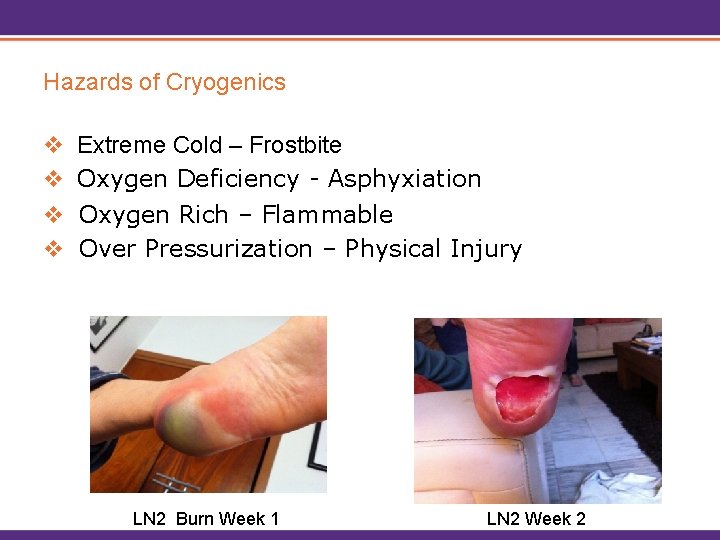
Hazards of Cryogenics v Extreme Cold – Frostbite v Oxygen Deficiency - Asphyxiation v Oxygen Rich – Flammable v Over Pressurization – Physical Injury LN 2 Burn Week 1 LN 2 Week 2

Ways to Expose Yourself • Directly touching the liquid with your skin • Indirectly touching something cooled by the cryogenic liquid like a metal transfer line • Indirectly by exposing skin or eyes to the cold gas coming out of a pressure relief valve at the end of a transfer line

Extreme Cold Hazards • Cryogenic liquids and cold vapors can cause thermal burn injuries, frostbite. • Brief exposures may damage tissue. • Breathing extremely cold air may damage lungs. • Skin may stick to metal that is cooled by cryogenic liquids and when pulled away the skin may tear. • Non-metallic materials are also dangerous to touch at cryogenic temperatures.

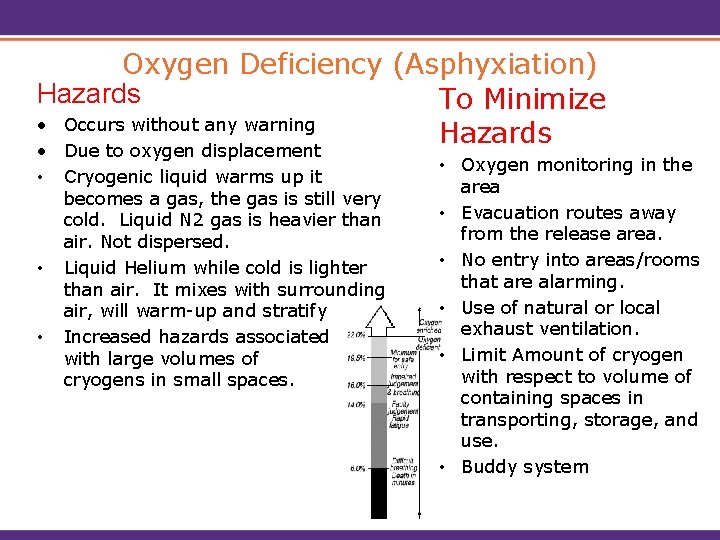
Oxygen Deficiency (Asphyxiation) Hazards To Minimize • Occurs without any warning Hazards • Due to oxygen displacement • Cryogenic liquid warms up it becomes a gas, the gas is still very cold. Liquid N 2 gas is heavier than air. Not dispersed. • Liquid Helium while cold is lighter than air. It mixes with surrounding air, will warm-up and stratify • Increased hazards associated with large volumes of cryogens in small spaces. • Oxygen monitoring in the area • Evacuation routes away from the release area. • No entry into areas/rooms that are alarming. • Use of natural or local exhaust ventilation. • Limit Amount of cryogen with respect to volume of containing spaces in transporting, storage, and use. • Buddy system

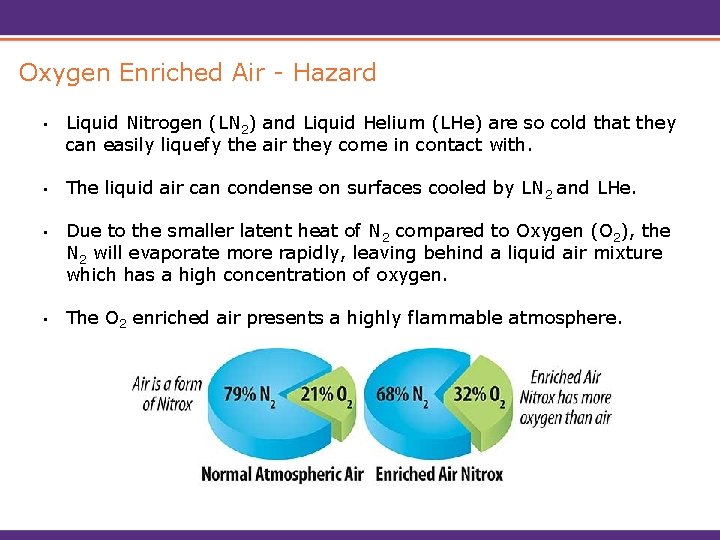
Oxygen Enriched Air - Hazard • Liquid Nitrogen (LN 2) and Liquid Helium (LHe) are so cold that they can easily liquefy the air they come in contact with. • The liquid air can condense on surfaces cooled by LN 2 and LHe. • Due to the smaller latent heat of N 2 compared to Oxygen (O 2), the N 2 will evaporate more rapidly, leaving behind a liquid air mixture which has a high concentration of oxygen. • The O 2 enriched air presents a highly flammable atmosphere.

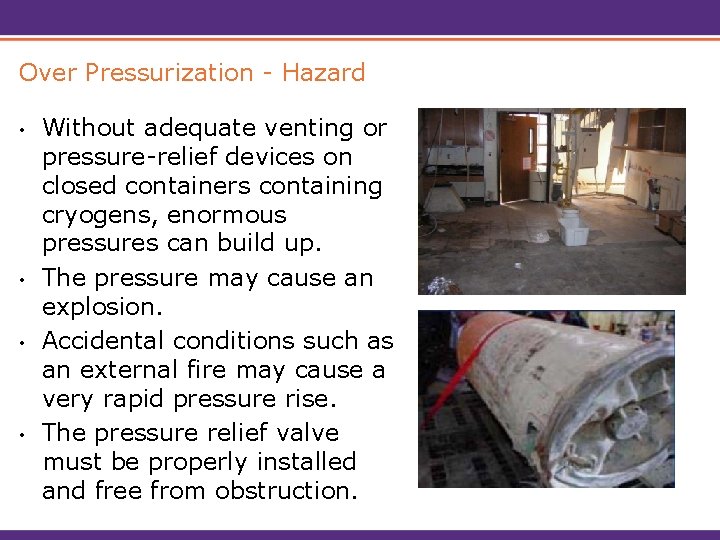
Over Pressurization - Hazard • • Without adequate venting or pressure-relief devices on closed containers containing cryogens, enormous pressures can build up. The pressure may cause an explosion. Accidental conditions such as an external fire may cause a very rapid pressure rise. The pressure relief valve must be properly installed and free from obstruction.

Dewar • Non pressurized container. • Typical capacity is a liter. • Product may be removed to smaller containers by pouring, but larger sizes require a transfer tube. • A loose fitting dust cap over the outlet prevents moisture from plugging the vent, allowing gas to escape. Signs of Damaged Dewar or Cryostat Continuous venting from a vent valve is not normal. It could mean there is dirt in the vent valve or it is damaged. Sweat or Frost at the bottom or sides of a dewar or cryostat is an indication of a faulty or damaged vacuum jacket.

Cryogen Safety Guidelines • Equipment should be kept clean. Perform routine inspections of all safety equipment and cryogenic systems. • Mixtures of gases or fluids should be strictly controlled to prevent formation of flammable or explosive mixtures. • Containers and systems containing cryogens should have pressure relief mechanisms. Ensure that all pressure relief valves and rupture disk vent paths are directed away from personnel. • Containers and systems should be capable of withstanding extreme cold without becoming brittle. Glass containers should be taped solidly around the outside or encased in plastic mesh. • Funnels should not be used for pouring liquid nitrogen or any other cryogen. Use a phase separator when transferring cryogens.

Cryogen Safety Guidelines • Use tongs or cryogenic gloves to handle charged liquid containers or other objects that might be cold. • Stay out of the path of boil off gases. • Pour cryogens slowly to minimize boiling and splashing. • Large mobile Dewars at risk for tipping should be transported on appropriate carts. Wheeled trolleys may not be used if the vessel must pass over elevator thresholds or other slots/crevasses wider than 25% of the wheel width.

Cryogen Safety Guidelines • Dispensing stations designed to allow research staff to fill smaller vessels from a larger self-pressurizing Dewar must be located in non-public areas, and should be posted with standard operating procedures. • Smaller vessels of liquid nitrogen or other cryogens transported by hand within or between buildings must have a handle or bail, and must be covered.

Personal Protective Equipment • Always wear goggles when handling cryogens. • If there is a splash or spray hazard, a face shield over the goggles. • An impervious apron or coat, cuff less long pants, and fullycovering shoes should be worn. • Watches, rings, and other jewelry should not be worn. Gloves should be impervious and sufficiently large to be readily thrown off should a cryogen be spilled. Cryo-gloves or pot holders should also be used.

Safe Handling of Dry Ice • Stored in a well-ventilated location. NEVER store dry ice in a cold room, warm room, or storage closet unless adequate supply ventilation is provided. • Do Not store in a tightly sealed device such as an ultra-low freezer, plastic/glass container, or other enclosed space. Store dry ice in a stryofoam chest, insulated cooler or a special cooler designed for the storage of dry ice (i. e. allows for the release of carbon dioxide gas). • NEVER handle dry ice with your bare hands. Always wear thermal gloves to reduce risk of thermal burns to the skin. • Do not place dry ice on a tiled or solid surface countertop as the extreme cold will cause damage. • Dry Ice is considered a Class 9 ( Hazardous material for shipping