CRYOELECTRON MICROSCOPY Presented By Rio Boothello Department of

CRYOELECTRON MICROSCOPY Presented By: Rio Boothello Department of Medicinal Chemistry Virginia Commonwealth University Email : boothellors@vcu. edu Date: 23 rd April 2010 1
STRUCTURAL INFORMATION http: //www. myfantasyweb. com/thumb/military. jpg (acessed on: 2
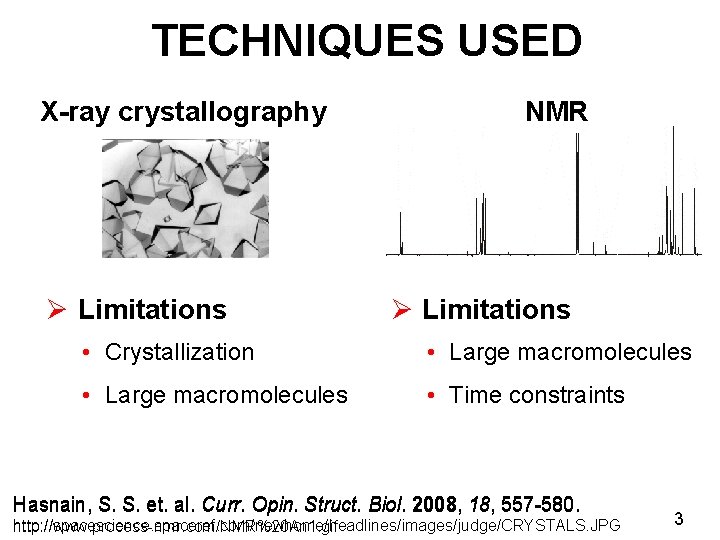
TECHNIQUES USED X-ray crystallography Ø Limitations NMR Ø Limitations • Crystallization • Large macromolecules • Time constraints Hasnain, S. S. et. al. Curr. Opin. Struct. Biol. 2008, 18, 557 -580. http: //spacescience. spaceref. com/newhome/headlines/images/judge/CRYSTALS. JPG http: //www. process-nmr. com/NMR%20 An 1. gif 3
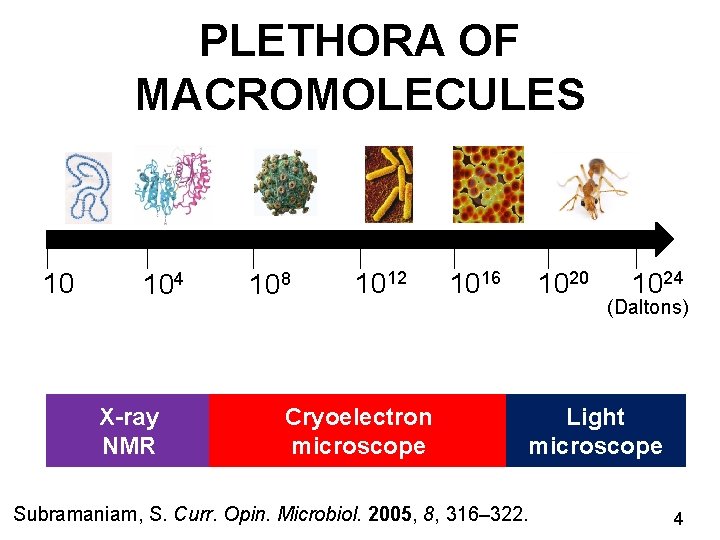
PLETHORA OF MACROMOLECULES 10 104 X-ray NMR 108 1012 Cryoelectron microscope 1016 1020 1024 (Daltons) Light microscope Subramaniam, S. Curr. Opin. Microbiol. 2005, 8, 316– 322. 4

HISTORY • 1674: Anton van Leeuwenhoek invented the first optical microscope • 1872: Ernst Abbe postulated an equation for resolution possible for a microscope Campbell, I. D. Nat. Rev. Mol. Cell Biol. 2002, 3, 377 -381. http: //www. scientific-web. com/en/Physics/Biographies/images/Ernst. Abbe 2. jpg h ttp: //www. findingdulcinea. com/docroot/dulcinea/fd_images/features/profiles/v/anton-vanleeuwenhoek/features/0/image. jpg accessed on 4/22/2010. acessed on : 4/22/2010. 5
DISCOVERY OF ELECTRON • 1897: J. J. Thomson discovered negatively charged particles called electron. § Low mass §Deflected by magnetic field. • 1924: Louis de Broglie postulated the wave -particle dual nature of an electron and the wavelength equation. Campbell, I. D. Nat. Rev. Mol. Cell Biol. 2002, 3, 377 -381. 6
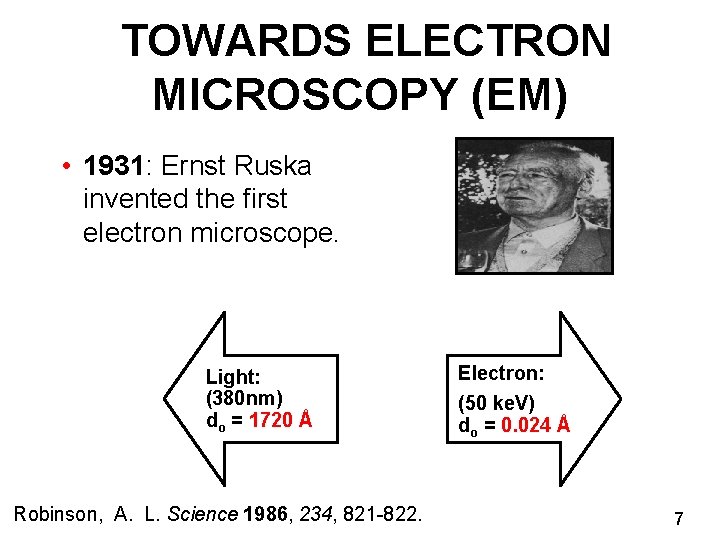
TOWARDS ELECTRON MICROSCOPY (EM) • 1931: Ernst Ruska invented the first electron microscope. Light: (380 nm) do = 1720 Å Robinson, A. L. Science 1986, 234, 821 -822. Electron: (50 ke. V) do = 0. 024 Å 7
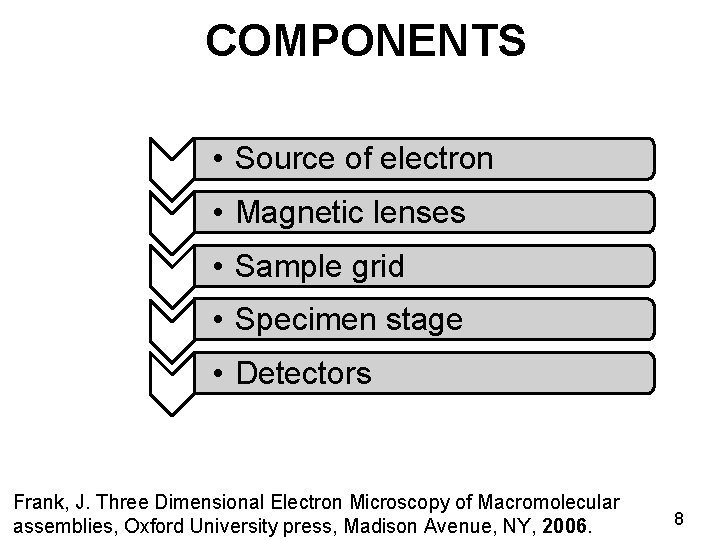
COMPONENTS • Source of electron • Magnetic lenses • Sample grid • Specimen stage • Detectors Frank, J. Three Dimensional Electron Microscopy of Macromolecular assemblies, Oxford University press, Madison Avenue, NY, 2006. 8
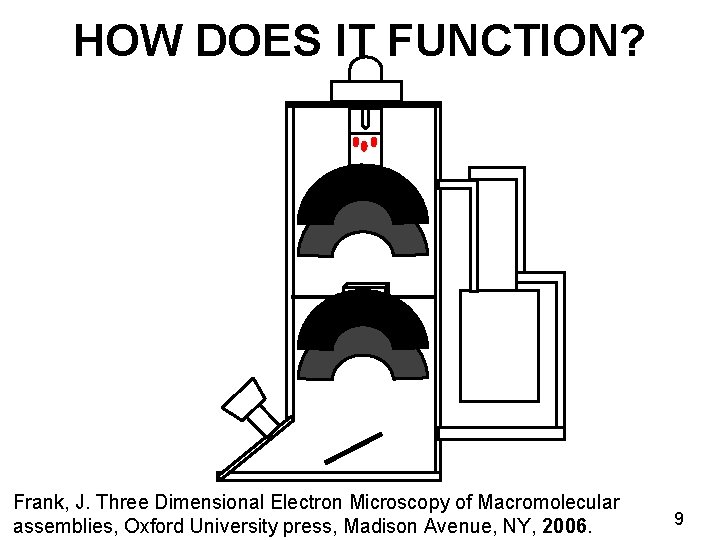
HOW DOES IT FUNCTION? Frank, J. Three Dimensional Electron Microscopy of Macromolecular assemblies, Oxford University press, Madison Avenue, NY, 2006. 9
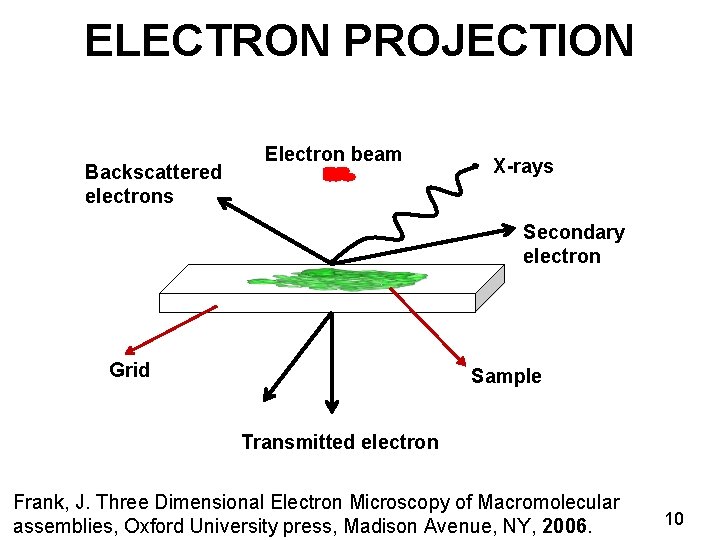
ELECTRON PROJECTION Backscattered electrons Electron beam X-rays Secondary electron Grid Sample Transmitted electron Frank, J. Three Dimensional Electron Microscopy of Macromolecular assemblies, Oxford University press, Madison Avenue, NY, 2006. 10
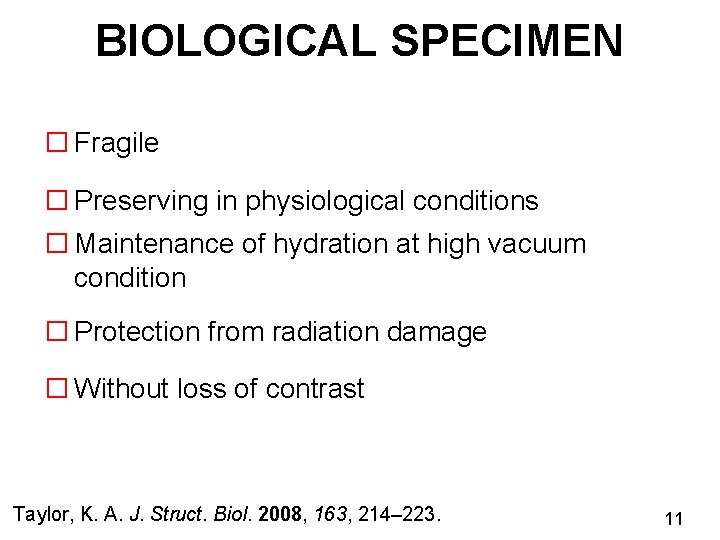
BIOLOGICAL SPECIMEN � Fragile � Preserving in physiological conditions � Maintenance of hydration at high vacuum condition � Protection from radiation damage � Without loss of contrast Taylor, K. A. J. Struct. Biol. 2008, 163, 214– 223. 11
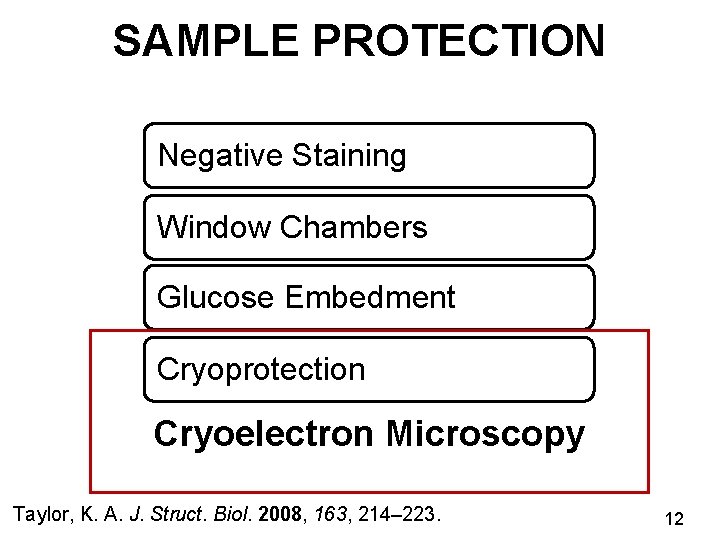
SAMPLE PROTECTION Negative Staining Window Chambers Glucose Embedment Cryoprotection Cryoelectron Microscopy Taylor, K. A. J. Struct. Biol. 2008, 163, 214– 223. 12
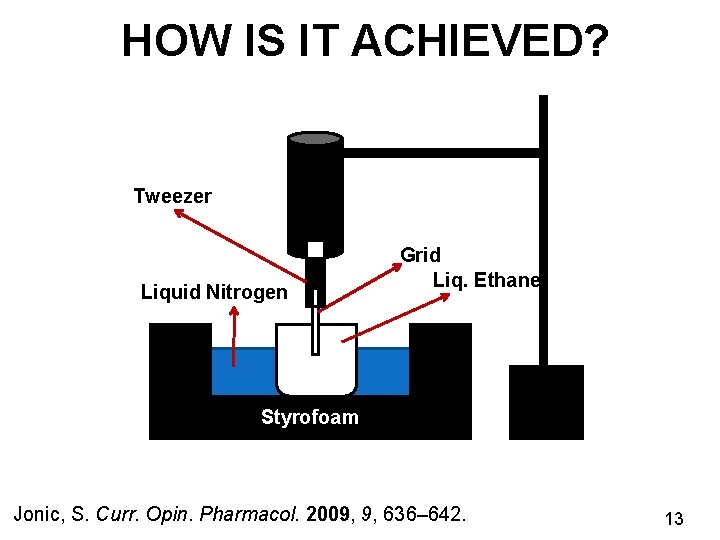
HOW IS IT ACHIEVED? Tweezer Liquid Nitrogen Grid Liq. Ethane Styrofoam Jonic, S. Curr. Opin. Pharmacol. 2009, 9, 636– 642. 13
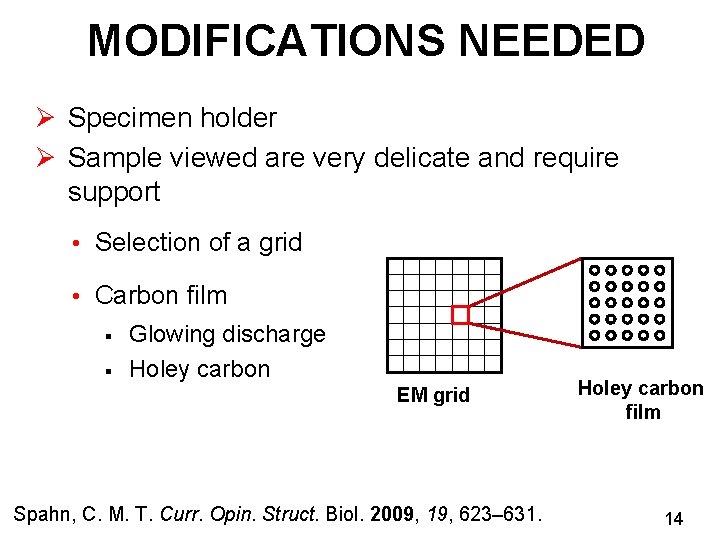
MODIFICATIONS NEEDED Ø Specimen holder Ø Sample viewed are very delicate and require support • Selection of a grid • Carbon film § § Glowing discharge Holey carbon EM grid Spahn, C. M. T. Curr. Opin. Struct. Biol. 2009, 19, 623– 631. Holey carbon film 14
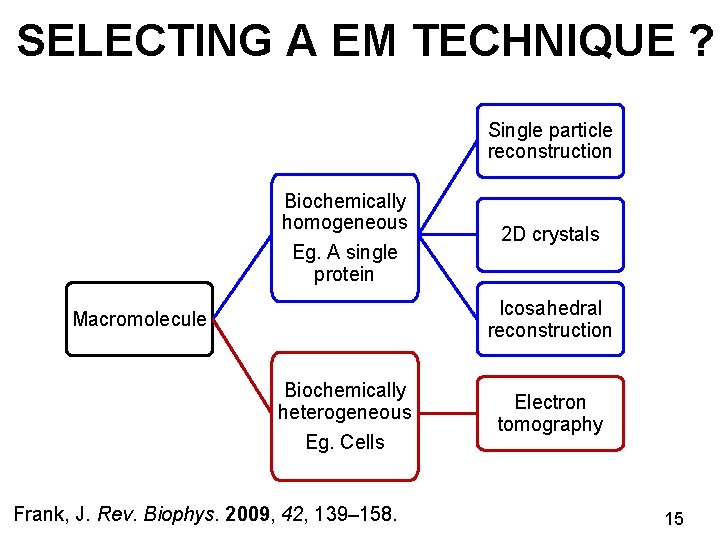
SELECTING A EM TECHNIQUE ? Single particle reconstruction Biochemically homogeneous Eg. A single protein 2 D crystals Icosahedral reconstruction Macromolecule Biochemically heterogeneous Eg. Cells Frank, J. Rev. Biophys. 2009, 42, 139– 158. Electron tomography 15
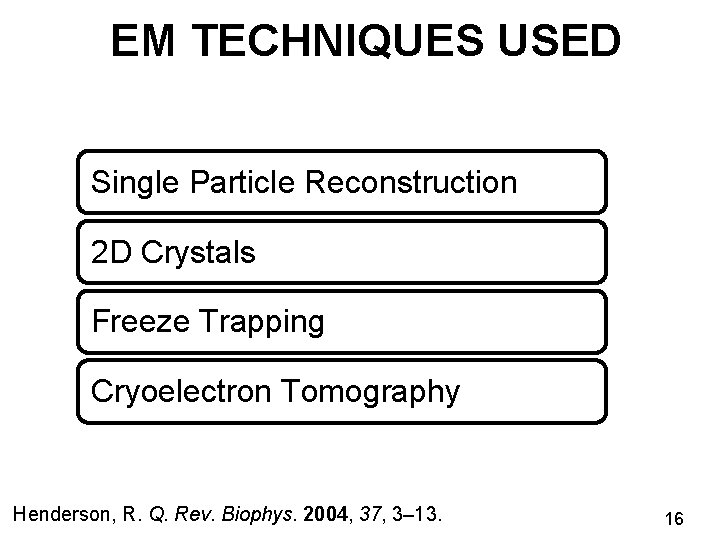
EM TECHNIQUES USED Single Particle Reconstruction 2 D Crystals Freeze Trapping Cryoelectron Tomography Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. 16
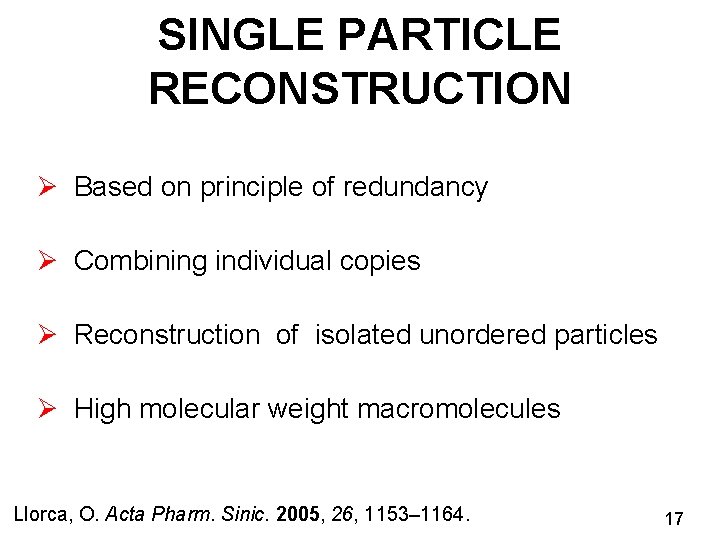
SINGLE PARTICLE RECONSTRUCTION Ø Based on principle of redundancy Ø Combining individual copies Ø Reconstruction of isolated unordered particles Ø High molecular weight macromolecules Llorca, O. Acta Pharm. Sinic. 2005, 26, 1153– 1164. 17
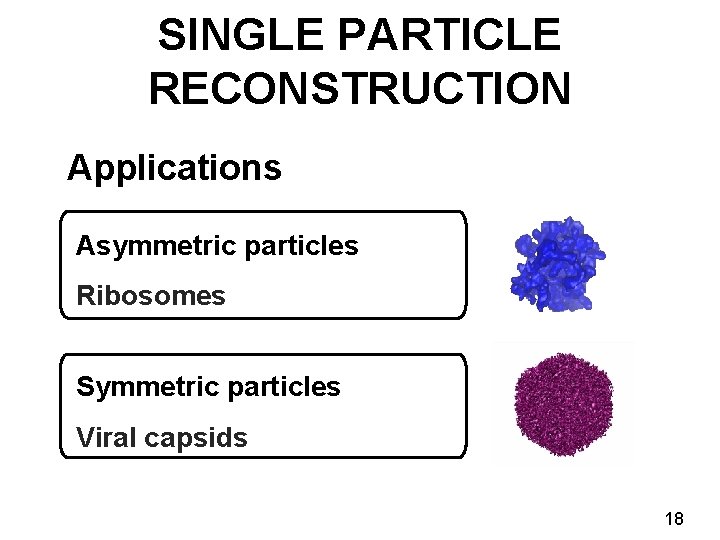
SINGLE PARTICLE RECONSTRUCTION Applications Asymmetric particles Ribosomes Symmetric particles Viral capsids 18
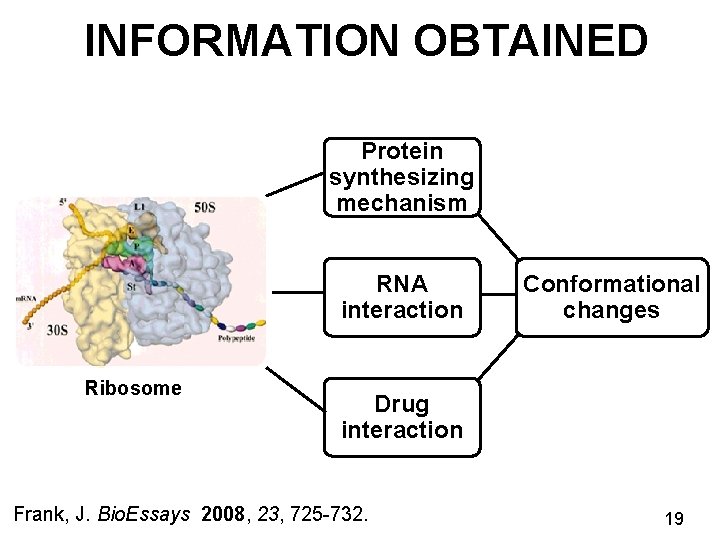
INFORMATION OBTAINED Protein synthesizing mechanism RNA interaction Ribosome Conformational changes Drug interaction Frank, J. Bio. Essays 2008, 23, 725 -732. 19
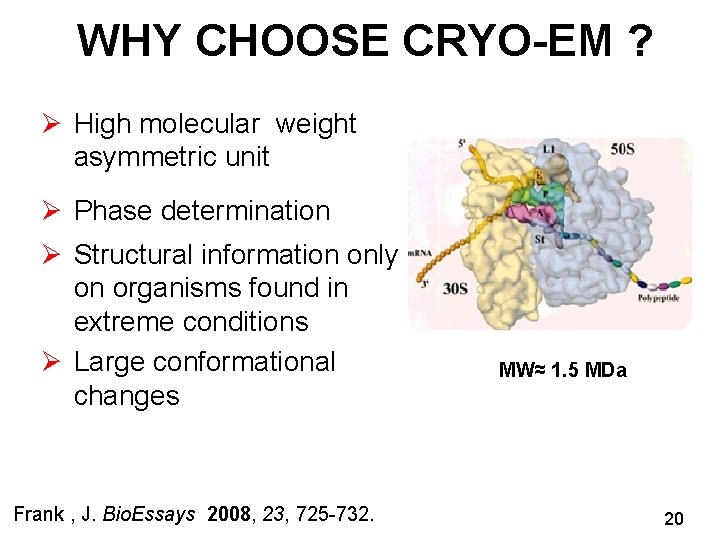
WHY CHOOSE CRYO-EM ? Ø High molecular weight asymmetric unit Ø Phase determination Ø Structural information only on organisms found in extreme conditions Ø Large conformational changes Frank , J. Bio. Essays 2008, 23, 725 -732. MW≈ 1. 5 MDa 20
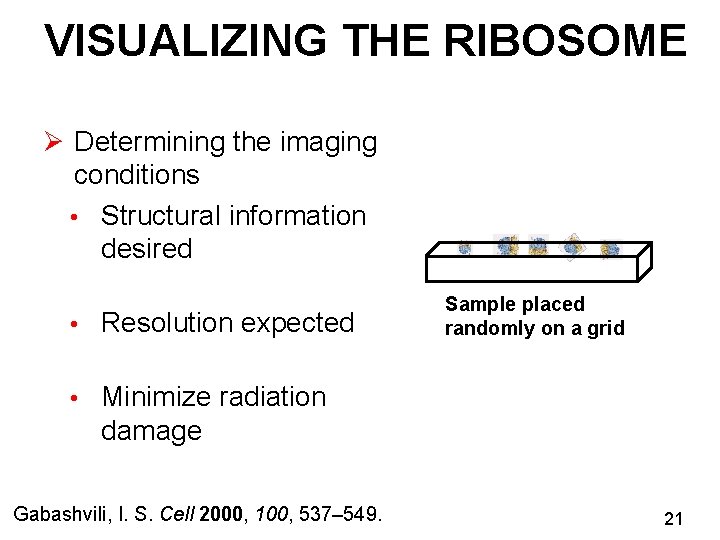
VISUALIZING THE RIBOSOME Ø Determining the imaging conditions • Structural information desired • Resolution expected Sample placed randomly on a grid • Minimize radiation damage Gabashvili, I. S. Cell 2000, 100, 537– 549. 21
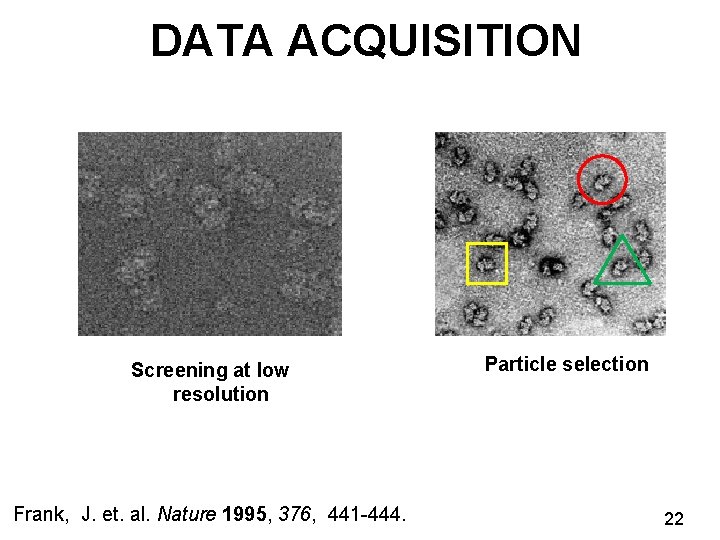
DATA ACQUISITION Screening at low resolution Frank, J. et. al. Nature 1995, 376, 441 -444. Particle selection 22
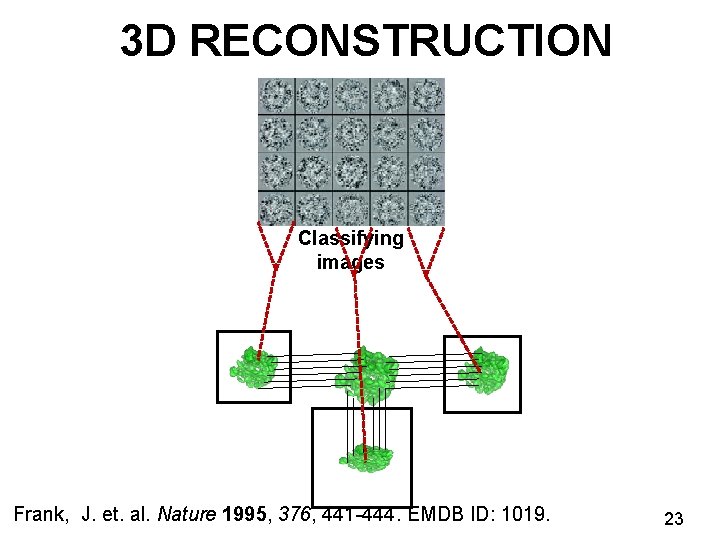
3 D RECONSTRUCTION Classifying images Frank, J. et. al. Nature 1995, 376, 441 -444. EMDB ID: 1019. 23
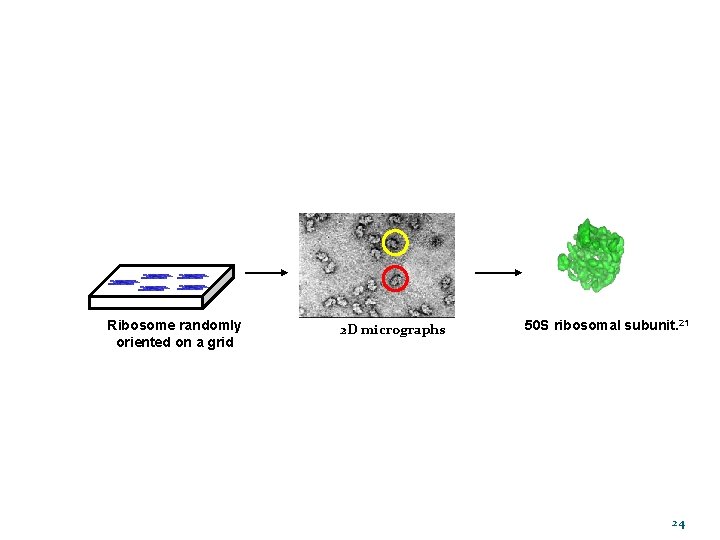
Ribosome randomly oriented on a grid 2 D micrographs 50 S ribosomal subunit. 21 24
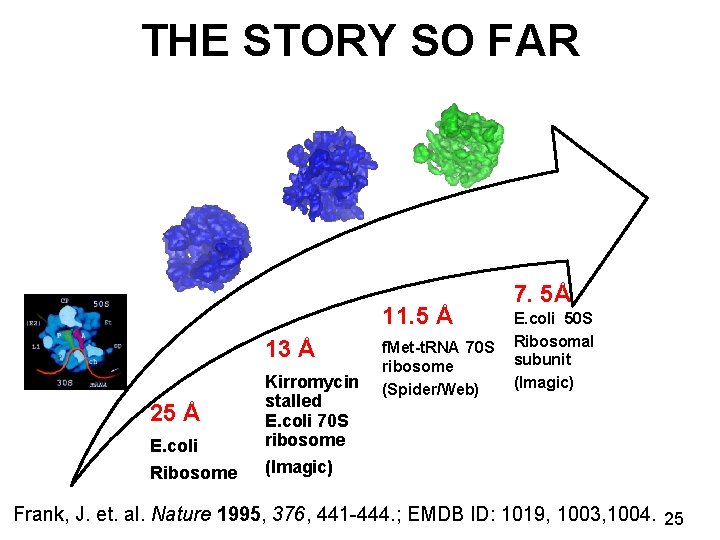
THE STORY SO FAR 11. 5 Å 13 Å 25 Å E. coli Ribosome Kirromycin stalled E. coli 70 S ribosome (Imagic) f. Met-t. RNA 70 S ribosome (Spider/Web) 7. 5Å E. coli 50 S Ribosomal subunit (Imagic) Frank, J. et. al. Nature 1995, 376, 441 -444. ; EMDB ID: 1019, 1003, 1004. 25
SINGLE PARTICLE RECONSTRUCTION Applications Asymmetric particles Ribosomes Symmetric particles Viral capsids 26
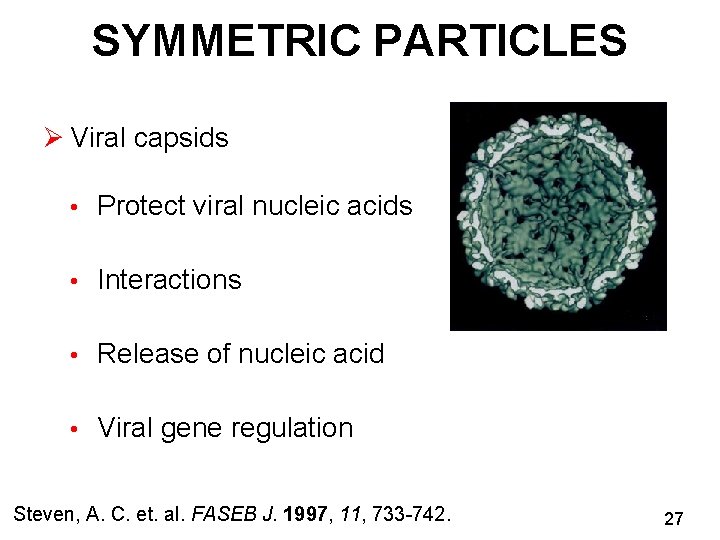
SYMMETRIC PARTICLES Ø Viral capsids • Protect viral nucleic acids • Interactions • Release of nucleic acid • Viral gene regulation Steven, A. C. et. al. FASEB J. 1997, 11, 733 -742. 27
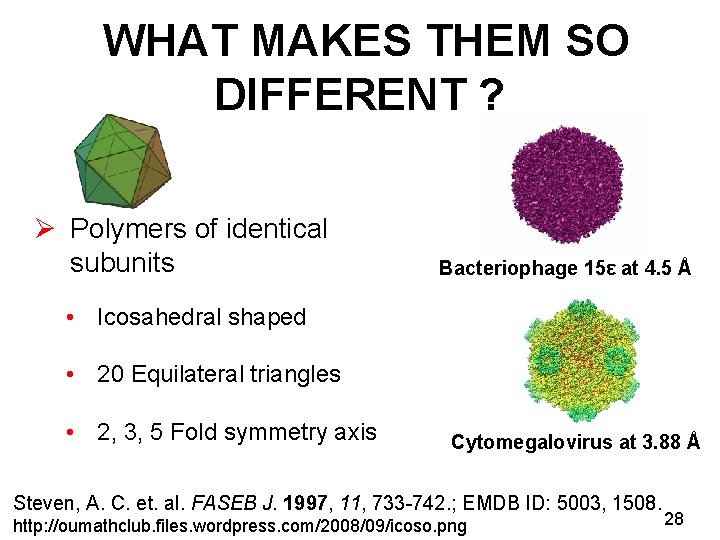
WHAT MAKES THEM SO DIFFERENT ? Ø Polymers of identical subunits Bacteriophage 15ε at 4. 5 Å • Icosahedral shaped • 20 Equilateral triangles • 2, 3, 5 Fold symmetry axis Cytomegalovirus at 3. 88 Å Steven, A. C. et. al. FASEB J. 1997, 11, 733 -742. ; EMDB ID: 5003, 1508. http: //oumathclub. files. wordpress. com/2008/09/icoso. png 28
EM TECHNIQUES USED Single Particle Reconstruction 2 D crystals Freeze trapping Cryoelectron tomography Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. 29
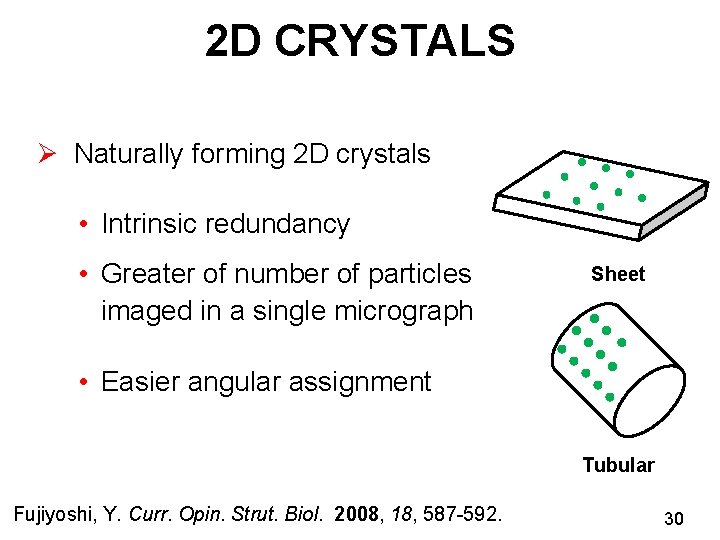
2 D CRYSTALS Ø Naturally forming 2 D crystals • Intrinsic redundancy • Greater of number of particles imaged in a single micrograph Sheet • Easier angular assignment Tubular Fujiyoshi, Y. Curr. Opin. Strut. Biol. 2008, 18, 587 -592. 30
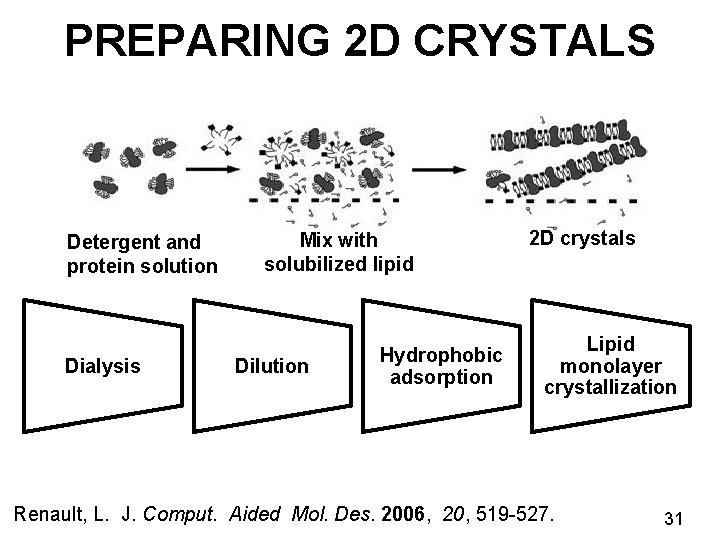
PREPARING 2 D CRYSTALS Detergent and protein solution Dialysis Mix with solubilized lipid Dilution Hydrophobic adsorption 2 D crystals Lipid monolayer crystallization Renault, L. J. Comput. Aided Mol. Des. 2006, 20, 519 -527. 31
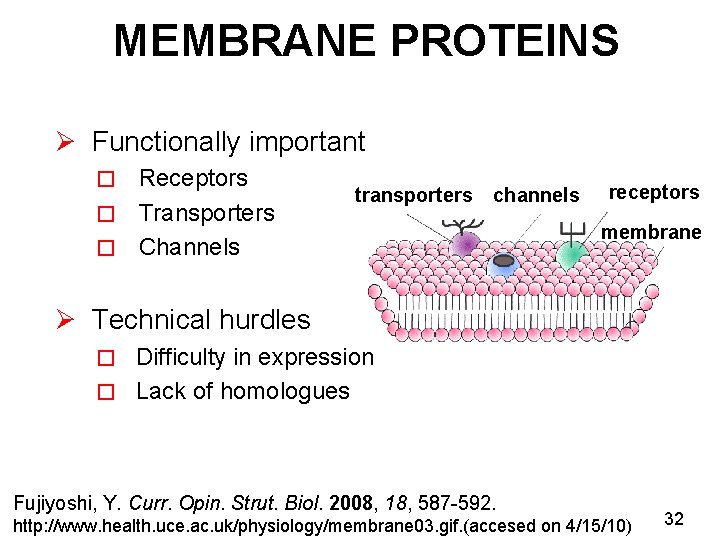
MEMBRANE PROTEINS Ø Functionally important Receptors � Transporters � Channels � transporters channels receptors membrane Ø Technical hurdles � Difficulty in expression � Lack of homologues Fujiyoshi, Y. Curr. Opin. Strut. Biol. 2008, 18, 587 -592. http: //www. health. uce. ac. uk/physiology/membrane 03. gif. (accesed on 4/15/10) 32
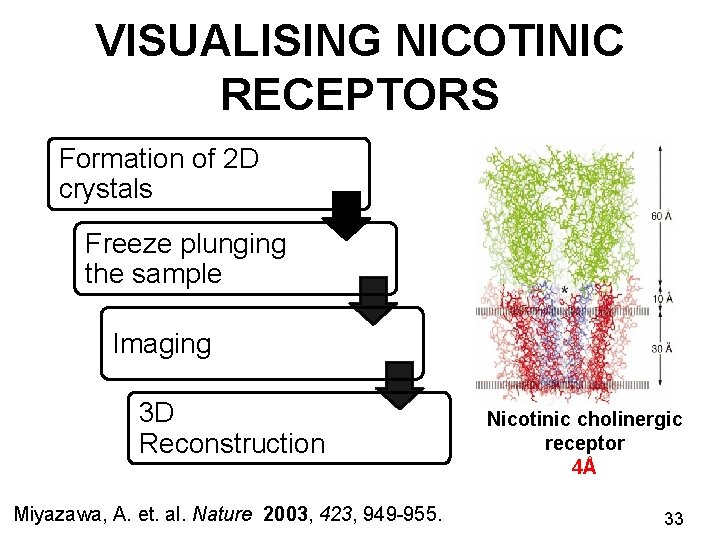
VISUALISING NICOTINIC RECEPTORS Formation of 2 D crystals Freeze plunging the sample Imaging 3 D Reconstruction Miyazawa, A. et. al. Nature 2003, 423, 949 -955. Nicotinic cholinergic receptor 4Å 33
TECHNIQUES USED Single Particle Reconstruction 2 D crystals Freeze trapping Cryoelectron tomography Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. 34
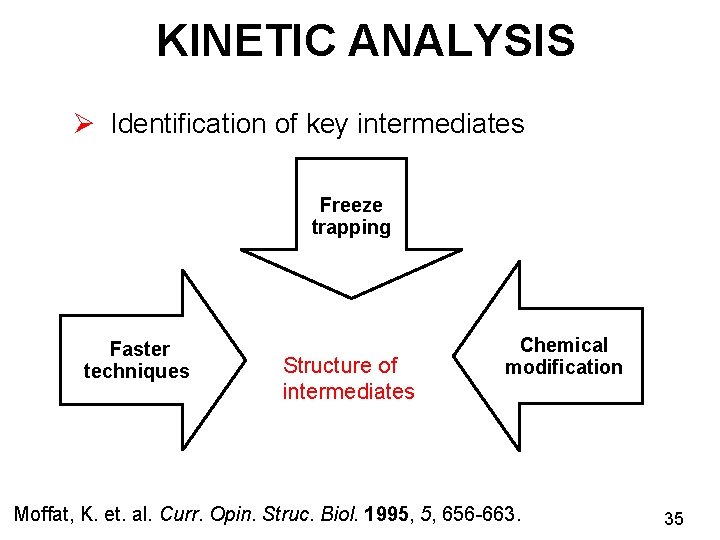
KINETIC ANALYSIS Ø Identification of key intermediates Freeze trapping Faster techniques Structure of intermediates Chemical modification Moffat, K. et. al. Curr. Opin. Struc. Biol. 1995, 5, 656 -663. 35
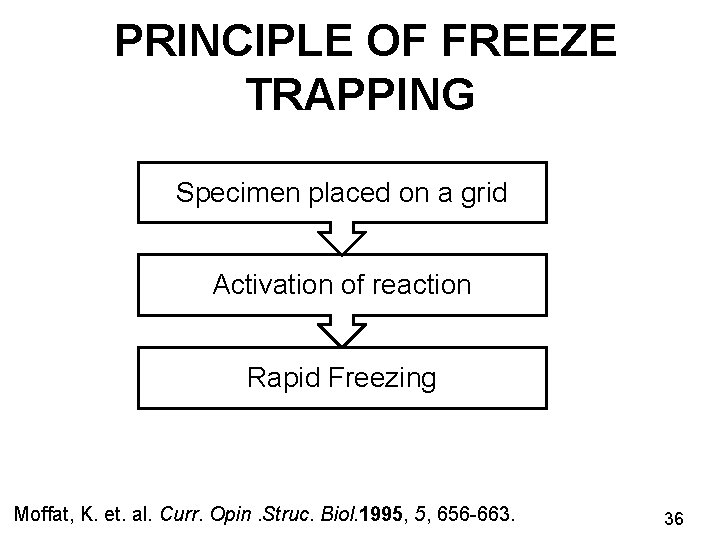
PRINCIPLE OF FREEZE TRAPPING Specimen placed on a grid Activation of reaction Rapid Freezing Moffat, K. et. al. Curr. Opin. Struc. Biol. 1995, 5, 656 -663. 36
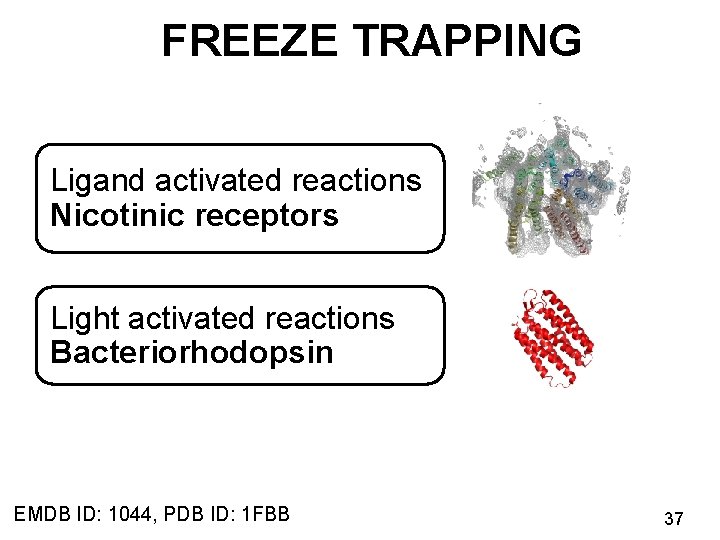
FREEZE TRAPPING Ligand activated reactions Nicotinic receptors Light activated reactions Bacteriorhodopsin EMDB ID: 1044, PDB ID: 1 FBB 37
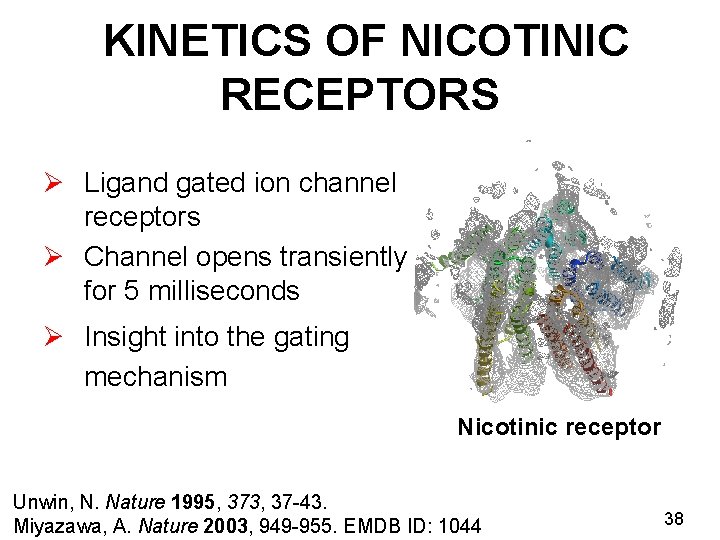
KINETICS OF NICOTINIC RECEPTORS Ø Ligand gated ion channel receptors Ø Channel opens transiently for 5 milliseconds Ø Insight into the gating mechanism Nicotinic receptor Unwin, N. Nature 1995, 373, 37 -43. Miyazawa, A. Nature 2003, 949 -955. EMDB ID: 1044 38
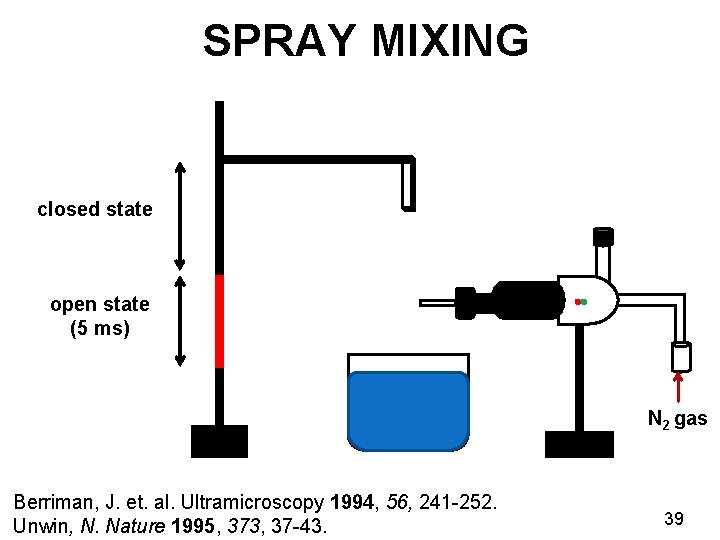
SPRAY MIXING closed state open state (5 ms) N 2 gas Berriman, J. et. al. Ultramicroscopy 1994, 56, 241 -252. Unwin, N. Nature 1995, 373, 37 -43. 39
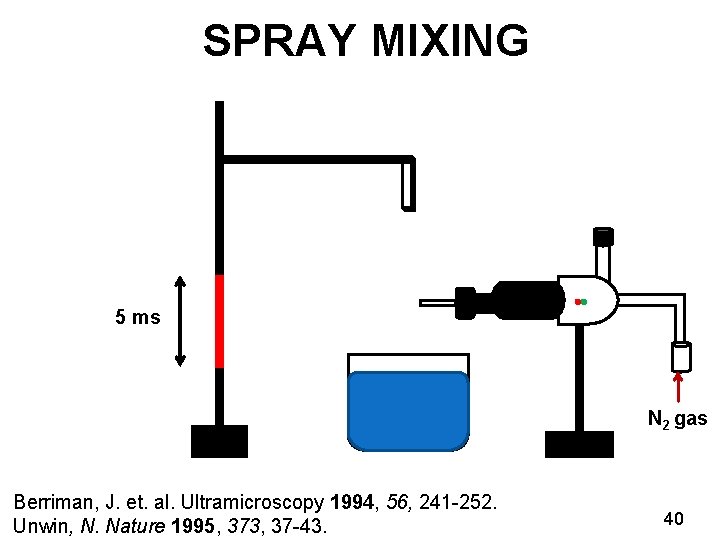
SPRAY MIXING 5 ms N 2 gas Berriman, J. et. al. Ultramicroscopy 1994, 56, 241 -252. Unwin, N. Nature 1995, 373, 37 -43. 40
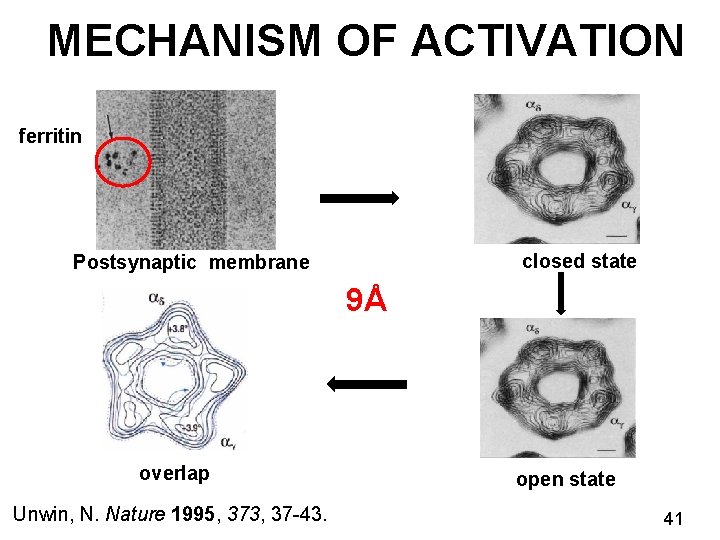
MECHANISM OF ACTIVATION ferritin closed state Postsynaptic membrane 9Å overlap Unwin, N. Nature 1995, 373, 37 -43. open state 41
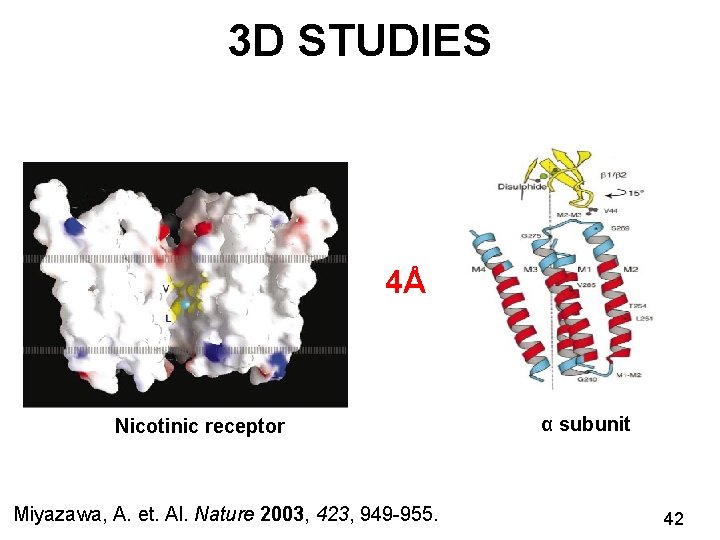
3 D STUDIES 4Å Nicotinic receptor Miyazawa, A. et. Al. Nature 2003, 423, 949 -955. α subunit 42
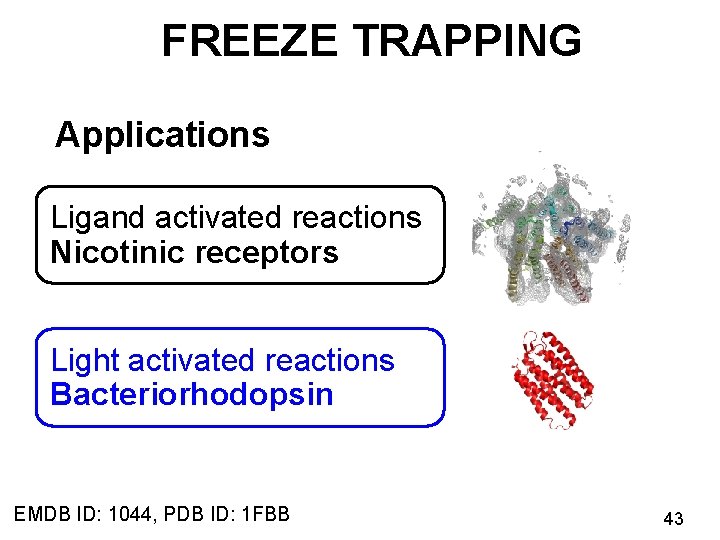
FREEZE TRAPPING Applications Ligand activated reactions Nicotinic receptors Light activated reactions Bacteriorhodopsin EMDB ID: 1044, PDB ID: 1 FBB 43
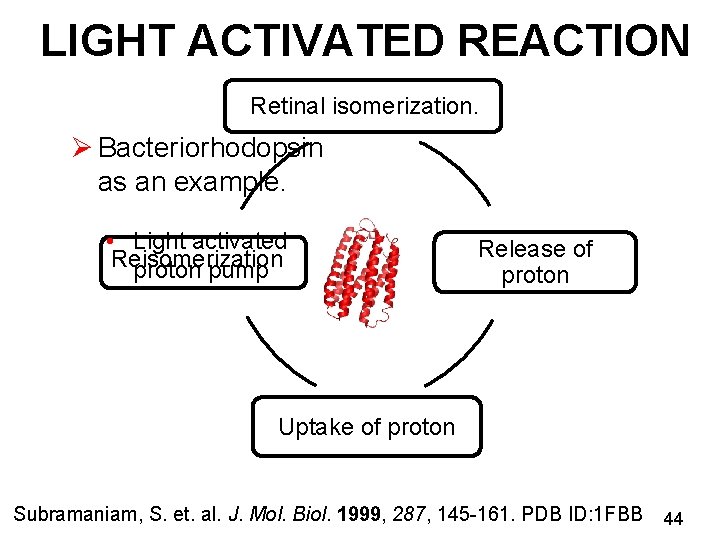
LIGHT ACTIVATED REACTION Retinal isomerization. Ø Bacteriorhodopsin as an example. • Light activated Reisomerization proton pump Release of proton Uptake of proton Subramaniam, S. et. al. J. Mol. Biol. 1999, 287, 145 -161. PDB ID: 1 FBB 44
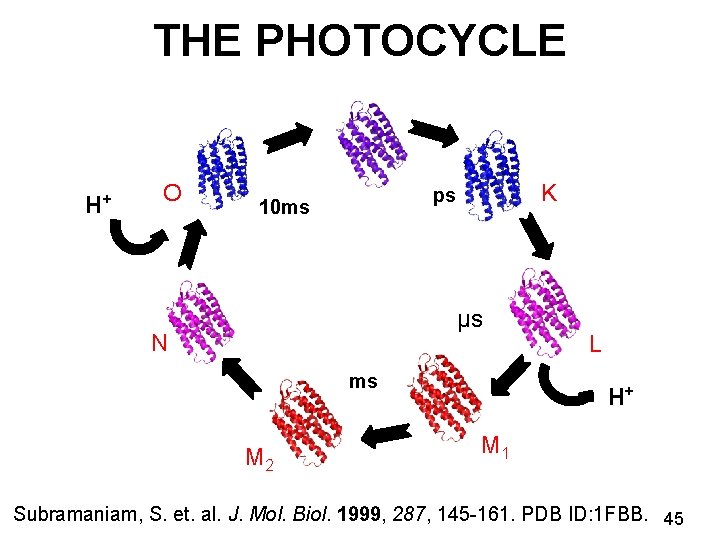
THE PHOTOCYCLE H+ O K ps 10 ms μs N ms M 2 L H+ M 1 Subramaniam, S. et. al. J. Mol. Biol. 1999, 287, 145 -161. PDB ID: 1 FBB. 45
EM TECHNIQUES USED Single Particle Reconstruction 2 D crystals Freeze trapping Cryoelectron tomography Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. 46
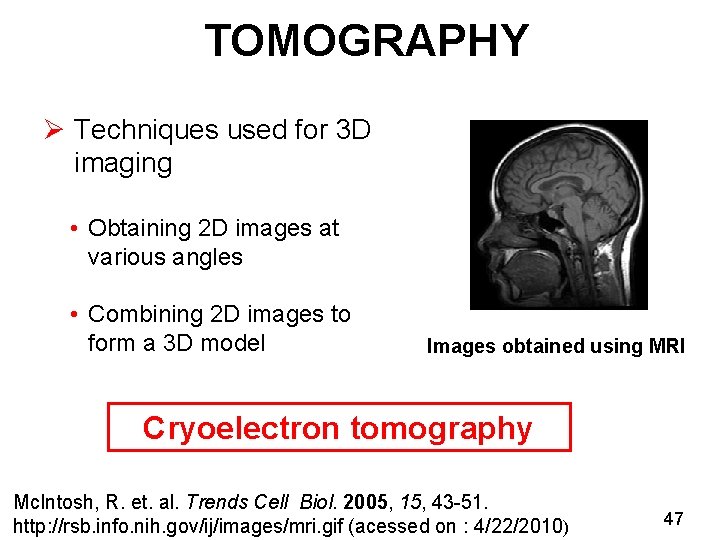
TOMOGRAPHY Ø Techniques used for 3 D imaging • Obtaining 2 D images at various angles • Combining 2 D images to form a 3 D model Images obtained using MRI Cryoelectron tomography Mc. Intosh, R. et. al. Trends Cell Biol. 2005, 15, 43 -51. http: //rsb. info. nih. gov/ij/images/mri. gif (acessed on : 4/22/2010) 47
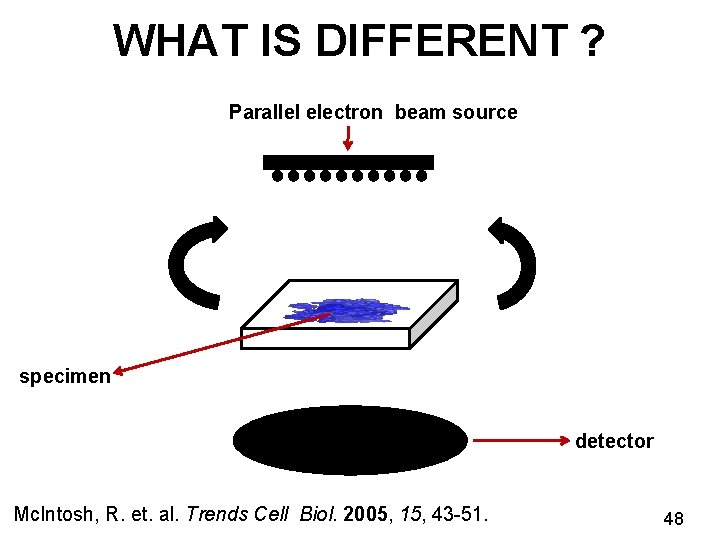
WHAT IS DIFFERENT ? Parallel electron beam source specimen detector Mc. Intosh, R. et. al. Trends Cell Biol. 2005, 15, 43 -51. 48
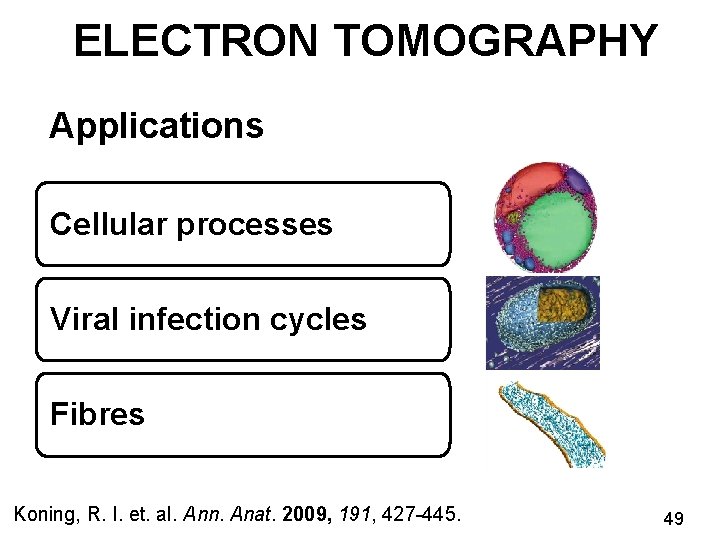
ELECTRON TOMOGRAPHY Applications Cellular processes Viral infection cycles Fibres Koning, R. I. et. al. Ann. Anat. 2009, 191, 427 -445. 49
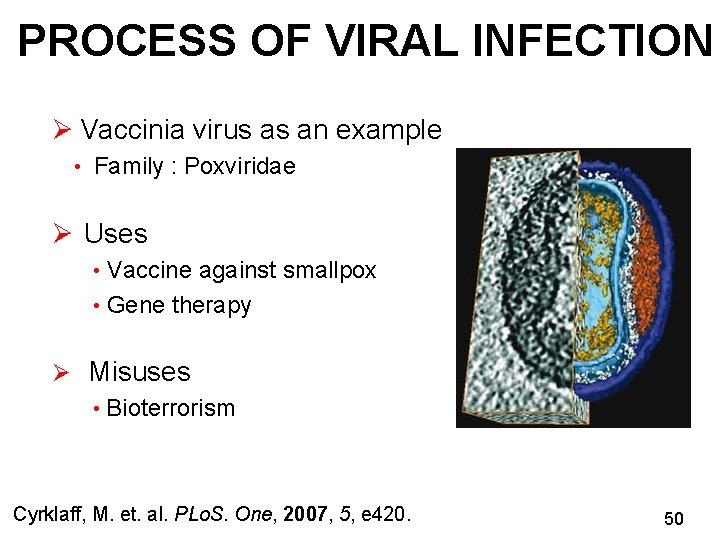
PROCESS OF VIRAL INFECTION Ø Vaccinia virus as an example • Family : Poxviridae Ø Uses • Vaccine against smallpox • Gene therapy Ø Misuses • Bioterrorism Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. 50
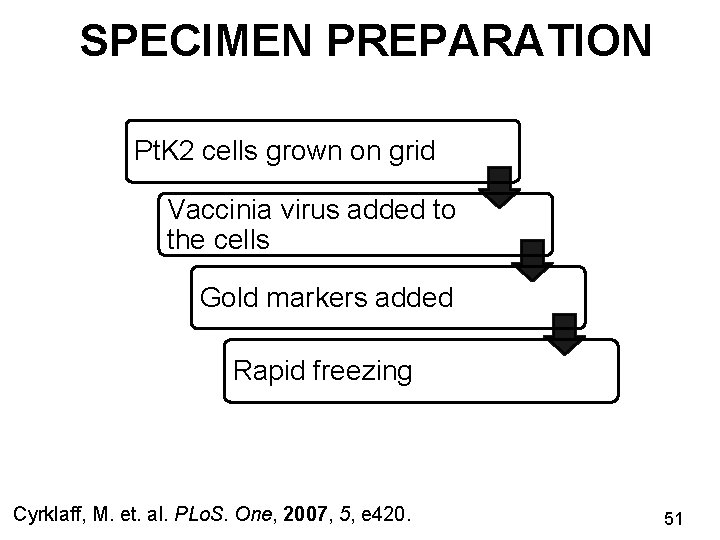
SPECIMEN PREPARATION Pt. K 2 cells grown on grid Vaccinia virus added to the cells Gold markers added Rapid freezing Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. 51
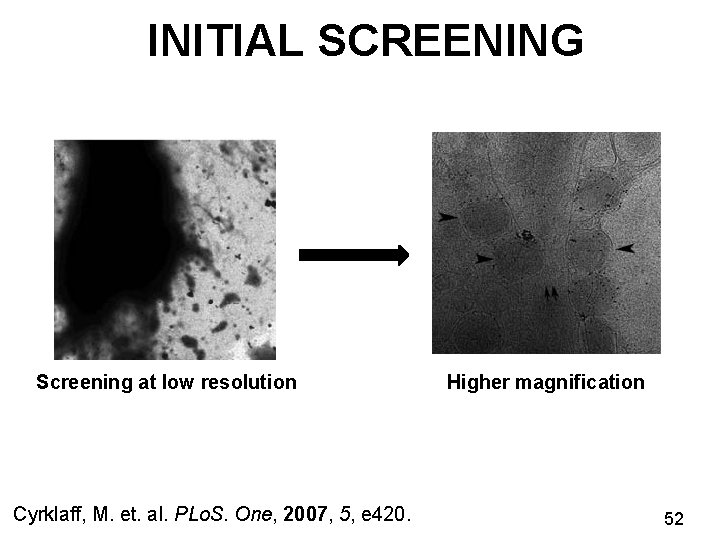
INITIAL SCREENING Screening at low resolution Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. Higher magnification 52
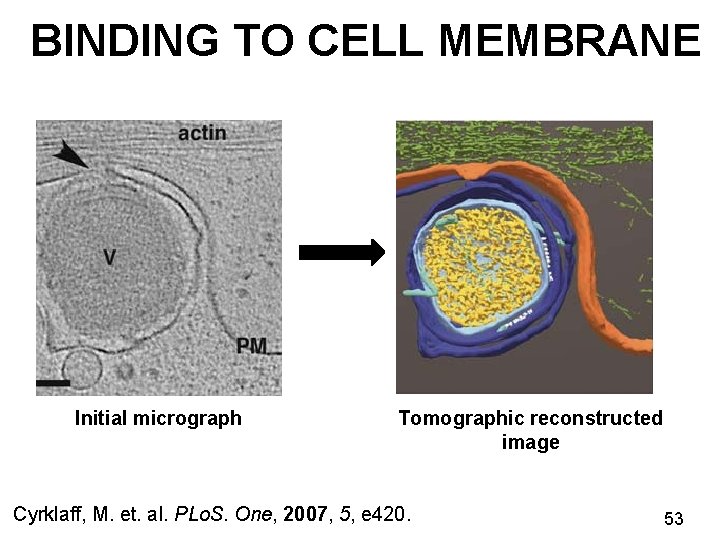
BINDING TO CELL MEMBRANE Initial micrograph Tomographic reconstructed image Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. 53
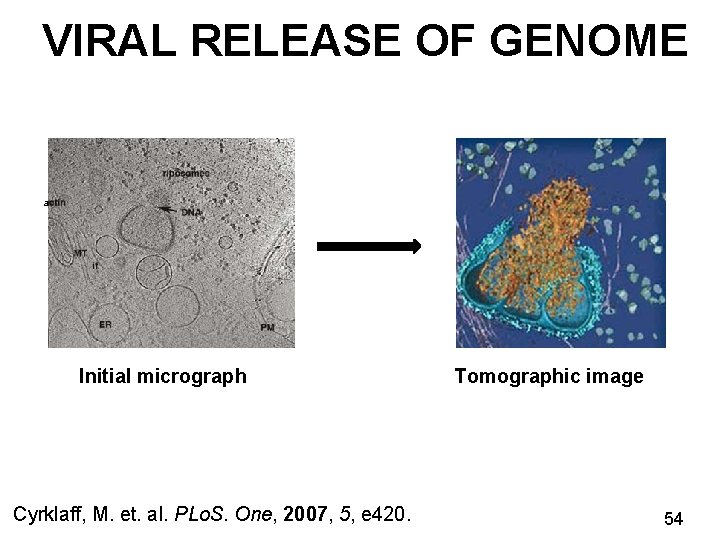
VIRAL RELEASE OF GENOME a Initial micrograph Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. Tomographic image 54
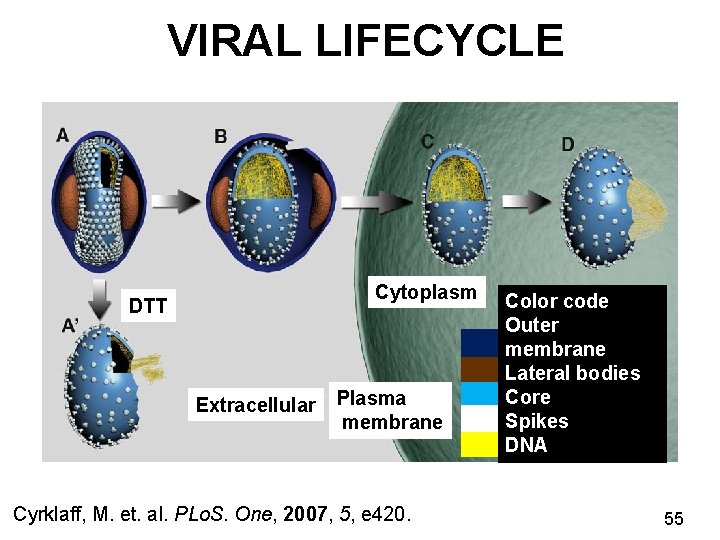
VIRAL LIFECYCLE Cytoplasm DTT Extracellular Plasma membrane Cyrklaff, M. et. al. PLo. S. One, 2007, 5, e 420. Color code Outer membrane Lateral bodies Core Spikes DNA 55
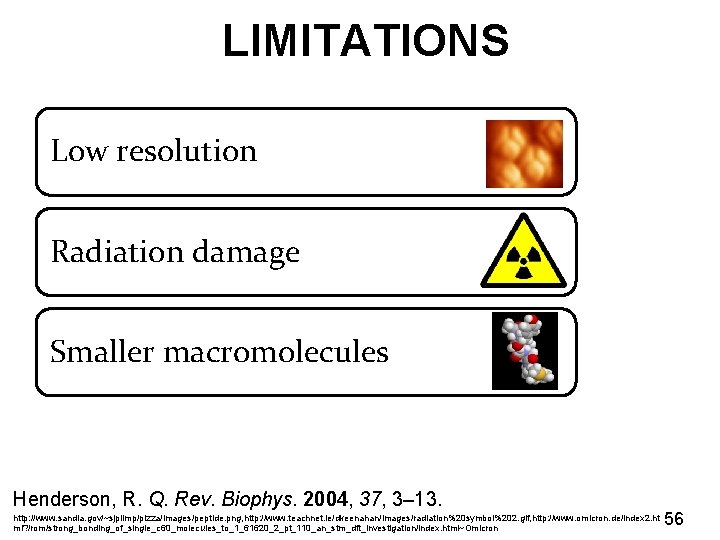
LIMITATIONS Low resolution Radiation damage Smaller macromolecules Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. http: //www. sandia. gov/~sjplimp/pizza/images/peptide. png, http: //www. teachnet. ie/dkeenahan/images/radiation%20 symbol%202. gif, http: //www. omicron. de/index 2. ht ml? /rom/strong_bonding_of_single_c 60_molecules_to_1_61620_2_pt_110_an_stm_dft_investigation/index. html~Omicron 56
THE FUTURE Ø Improvements • The electron microscope • 3 D Reconstruction techniques • Preservation techniques • High throughput methods Henderson, R. Q. Rev. Biophys. 2004, 37, 3– 13. 57
SUMMARY Many forms of specimen • Large molecular Heterogeneous samples weight • Asymmetric unitsobtained Phase information • • Cellular Crystalsorganization • Virus lifecycle • Used to solvestudies phase problem in Time resolved solving crystal structure • Ligand activated More efficient • Light activated • p. H dependent Frank, J. Rev. Biophys. 2009, 42, 139– 158. 58
ACKNOWLEDGEMENTS Ø Dr. Umesh Desai Ø Dr. Tonie Wright Ø The Desai group Ø The Department of Medicinal Chemistry at VCU http: //img. directindustry. com/images_di/photo-g/transmission-electron-microscope-tem 105806. jpg 59
- Slides: 59