CRUSADE A National Quality Improvement Initiative Can Rapid











- Slides: 15

CRUSADE: A National Quality Improvement Initiative Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation of the ACC/AHA Guidelines

CRUSADE Description n CRUSADE is a Quality Improvement Initiative designed to improve the care of high-risk patients with NSTE ACS ¾ by collecting data regarding patient management practice patterns in the U. S. and ¾ using those data to target educational interventions designed to promote adherence to the revised ACC/AHA NSTE ACS guidelines recommendations.

CRUSADE Objectives n Determine the current state of awareness of and adherence to the ACC/AHA Non-ST-segment Elevation Acute Coronary Syndromes (NSTE ACS) Guidelines. n Implement quality improvement initiatives to promote ACC/AHA NSTE ACS Guidelines recommendations. n Improve clinical outcomes for NSTE ACS patients via early risk stratification and implementation of evidence-based care, both in-hospital and post-discharge.

ACC/AHA Treatment Recommendations Acute Therapy n n n Aspirin n Clopidogrel/ticlopidine, if contraindicated Beta blocker n Ca 2+ blocker , if contraindicated ACE inhibitor (HTN, CHF) Heparin (UFH or LMWH) GP IIb-IIIa inhibitor n All high-risk patients n All receiving PCI n Those with recurring ischemia Discharge Therapy n Aspirin n Beta blocker n Lipid-lowering agent/statin n ACE inhibitor n Cardiac rehabilitation n Smoking cessation

CRUSADE Design n Nationwide Quality Improvement (QI) initiative n Up to 600 participating hospitals n Collaborative effort between Emergency Medicine, Cardiology, Hospital QI, Academia, and Industry n Focused on improving the care of NSTE ACS patients

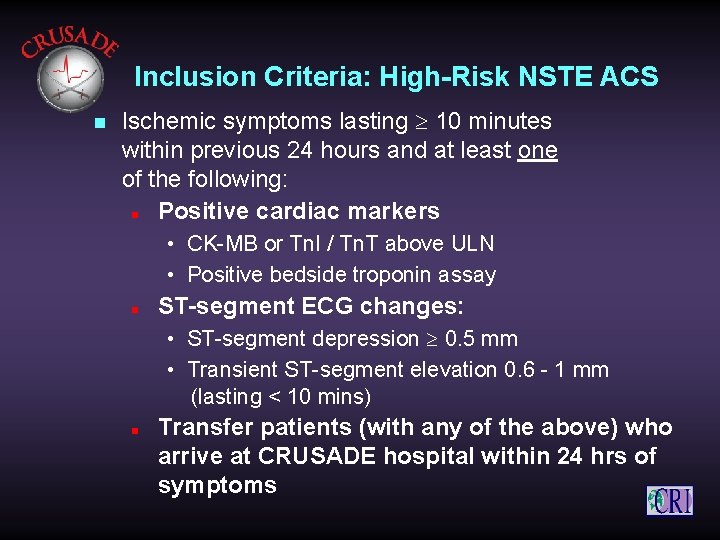
Inclusion Criteria: High-Risk NSTE ACS n Ischemic symptoms lasting 10 minutes within previous 24 hours and at least one of the following: n Positive cardiac markers • CK-MB or Tn. I / Tn. T above ULN • Positive bedside troponin assay n ST-segment ECG changes: • ST-segment depression 0. 5 mm • Transient ST-segment elevation 0. 6 - 1 mm (lasting < 10 mins) n Transfer patients (with any of the above) who arrive at CRUSADE hospital within 24 hrs of symptoms

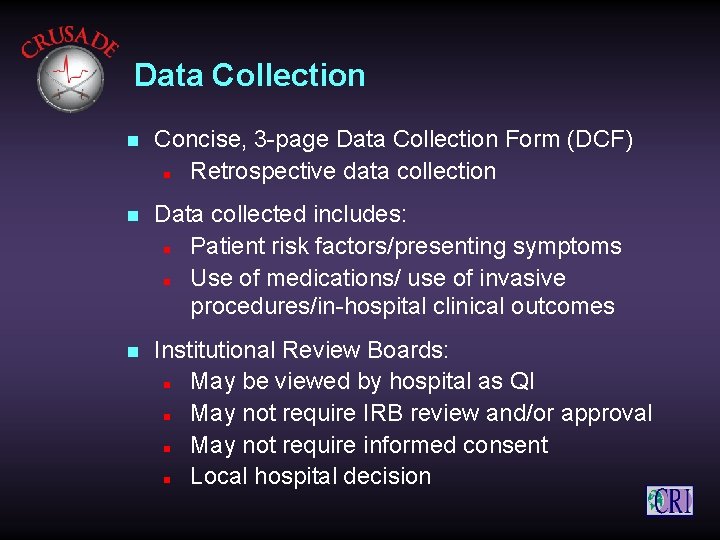
Data Collection n Concise, 3 -page Data Collection Form (DCF) n Retrospective data collection n Data collected includes: n Patient risk factors/presenting symptoms n Use of medications/ use of invasive procedures/in-hospital clinical outcomes n Institutional Review Boards: n May be viewed by hospital as QI n May not require IRB review and/or approval n May not require informed consent n Local hospital decision

Quality Improvement Initiative: Measuring Change n Effectiveness of QI initiatives measured by changes in adherence to ACC/AHA treatment Guidelines n Early / discharge aspirin use n Early / discharge beta blocker use n Discharge ACE inhibitor and statin use n GP IIb-IIIa inhibitors: early use and use during PCI n Appropriate secondary prevention measures • Smoking cessation • Cardiac rehabilitation

Quality Improvement Initiatives: Data Reporting to Sites n Quarterly feedback reports to sites regarding their adherence to ACC/AHA Guidelines n n n Focused on the ACC/AHA Guidelines treatment & management recommendations Site confidentiality maintained—data supplied back to sites in a blinded fashion Provides sites with benchmark performance data

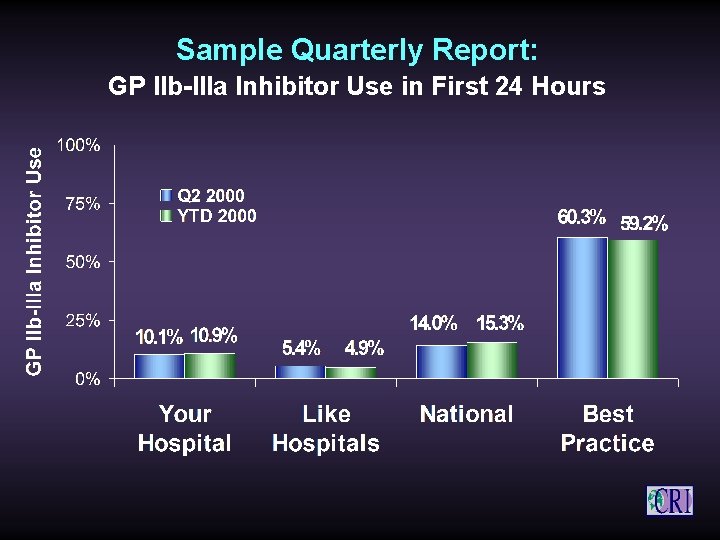
Sample Quarterly Report: GP IIb-IIIa Inhibitor Use in First 24 Hours

Sample Quarterly Report: Discharge Beta Blocker Use

Benefits of Participation n Quality improvement performance feedback n n Reports documenting utilization of evidence-based management strategies and therapies. Performance ranking among “like”, national, and “best practice” hospitals. Insight into hospital care and areas for improvement. May be applied to hospital QI monitoring efforts/requirements.

Benefits of Participation n Quality improvement tools to help improve outcomes for high-risk NSTE ACS patients. n Initiatives to help increase understanding of the ACC/AHA Guidelines. n n Initiatives to improve risk stratification and diagnosis of NSTE ACS patients. Newsletters and website for continuing education.

Promoting a New Paradigm of Evidence-Based Cardiovascular Care n The CRUSADE national quality improvement initiative will teach us much about: n n Why current ACC/AHA Guidelines for ACS are not followed. What initiatives can improve adherence. How to promote Emergency Medicine Cardiology collaboration. Will improved early adherence to treatment guidelines lead to better acute outcomes.

CRUSADE Implementation n Training of staff and physicians should be conducted by CRUSADE co-advocates prior to the start of CRUSADE. n Participating hospitals must complete, sign, and return a CRUSADE Participation Agreement. n Data collection can then commence. n Quarterly, the site will receive a Feedback Report detailing use of Guidelines-recommended therapies.
 Mqii toolkit
Mqii toolkit Rapid cycle quality improvement
Rapid cycle quality improvement Quality assurance cycle in nursing
Quality assurance cycle in nursing Compliance vs quality
Compliance vs quality Rapid cycle improvement model
Rapid cycle improvement model Rapid process improvement workshop
Rapid process improvement workshop Rapid cycle improvement
Rapid cycle improvement Rapid process improvement methodology
Rapid process improvement methodology Rapid process improvement workshop
Rapid process improvement workshop Alicia cardell
Alicia cardell What is a crusade
What is a crusade Crusade themes
Crusade themes Propaganda movement members and their pen names
Propaganda movement members and their pen names The triumphs of a crusade chapter 21 section 2
The triumphs of a crusade chapter 21 section 2 Fourth crusade
Fourth crusade Crusades used in a sentence
Crusades used in a sentence