Crude oil Treatment process Hydrotreatment Amine recovery Sulfur

















- Slides: 54

Crude oil Treatment process Hydrotreatment Amine recovery Sulfur recovery 1

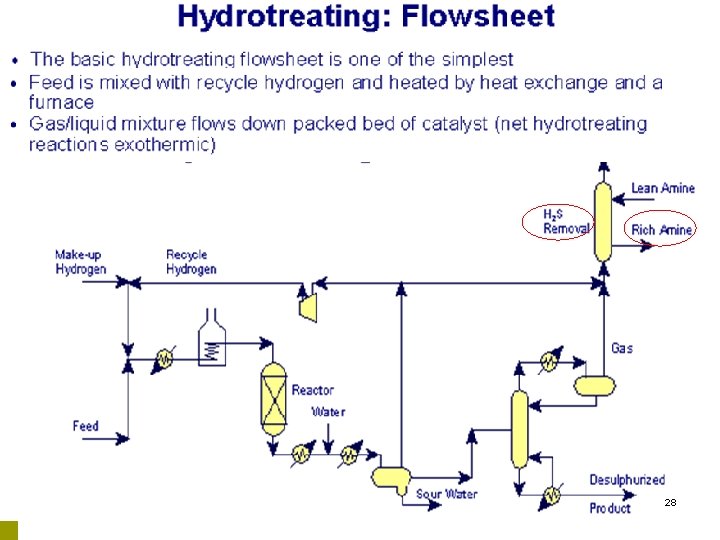
Hydrotreater BP-UK 2

Hydrotreatment What is hydrotreatment? p It is a process to catalytically stabilize and/or remove undesirable elements from products or feedstocks by reacting them with hydrogen. p Stabilization usually involves converting unsaturated hydrocarbons such as olefins to paraffins. p Undesirable elements removed by hydrotreating include sulfur, nitrogen, oxygen, halides, and metals. p 3

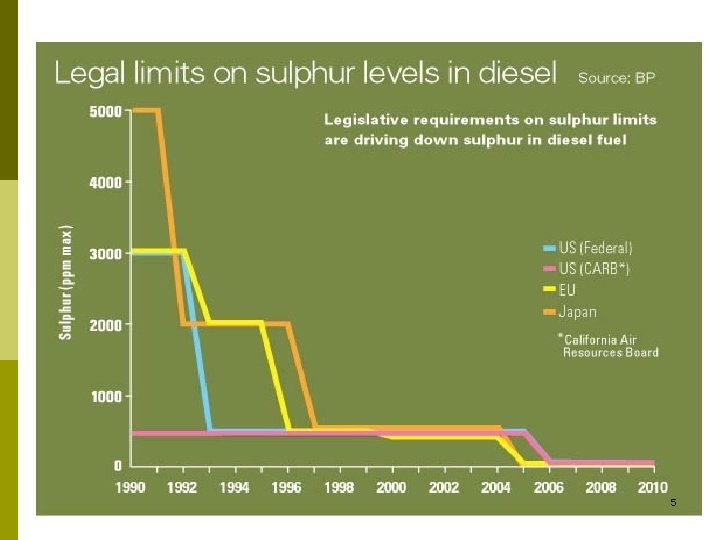
4

5

Difference between Hydrotreating and Hydrocracking Hydrotreating and hydrocracking both can be called Hydroprocessing 6

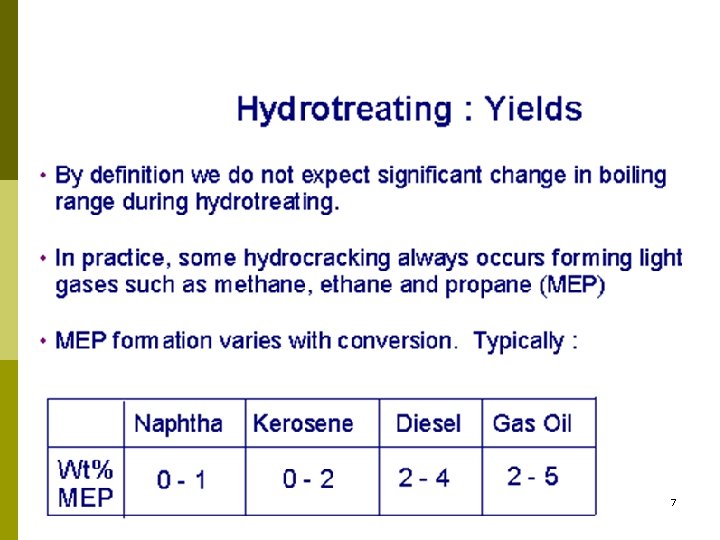
7

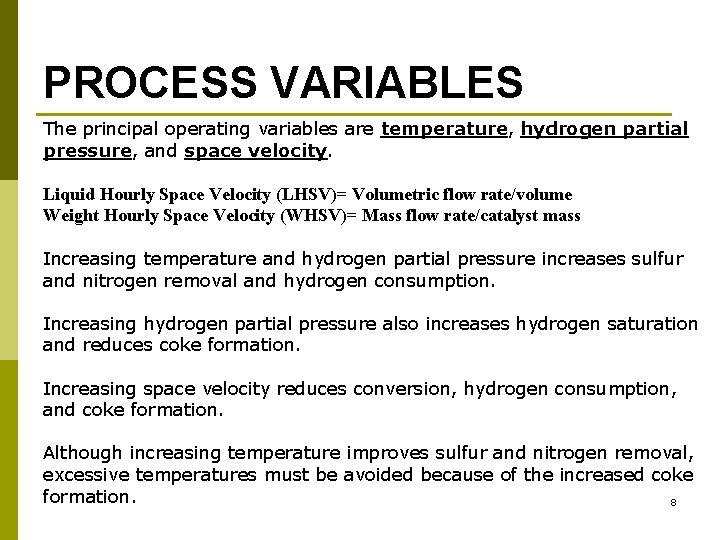
PROCESS VARIABLES The principal operating variables are temperature, hydrogen partial pressure, and space velocity. Liquid Hourly Space Velocity (LHSV)= Volumetric flow rate/volume Weight Hourly Space Velocity (WHSV)= Mass flow rate/catalyst mass Increasing temperature and hydrogen partial pressure increases sulfur and nitrogen removal and hydrogen consumption. Increasing hydrogen partial pressure also increases hydrogen saturation and reduces coke formation. Increasing space velocity reduces conversion, hydrogen consumption, and coke formation. Although increasing temperature improves sulfur and nitrogen removal, excessive temperatures must be avoided because of the increased coke formation. 8

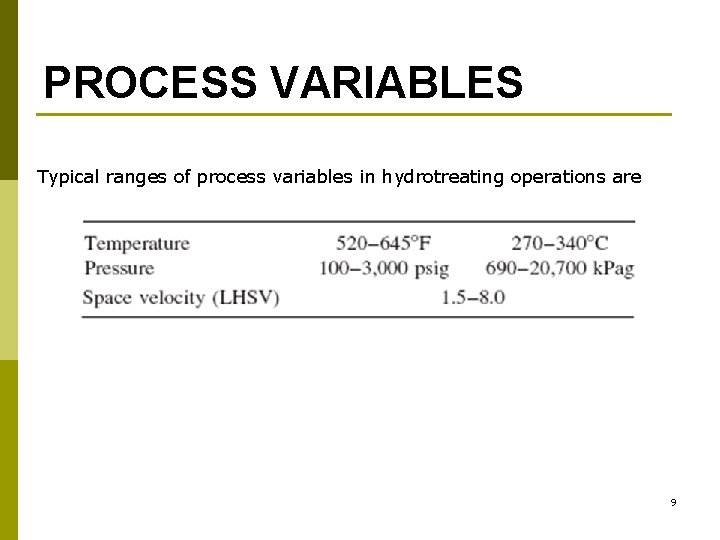
PROCESS VARIABLES Typical ranges of process variables in hydrotreating operations are 9

10

11

12

13

Hydrotreatent Reactions p Convert Organic S to Hydrogen Sulfide p Convert Organic N to Ammonia p Convert Organic O to Water p Convert Organic halides to Hydrogen Halides p Saturate Olefins p Saturate Aromatics p Remove Metals 14

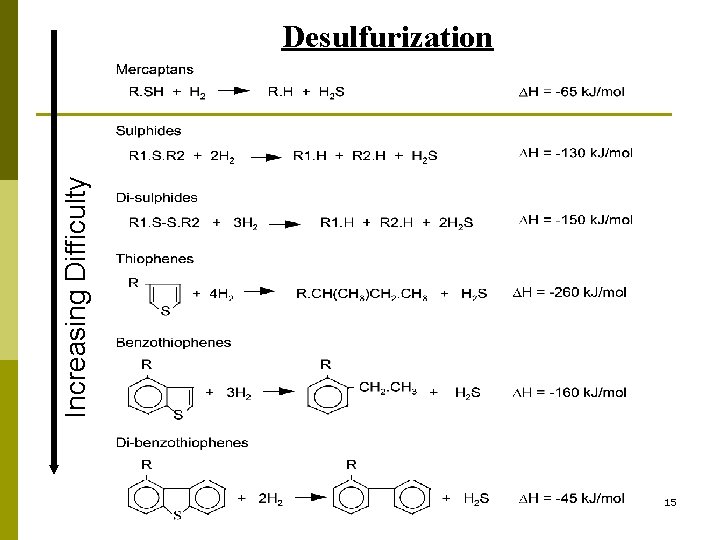
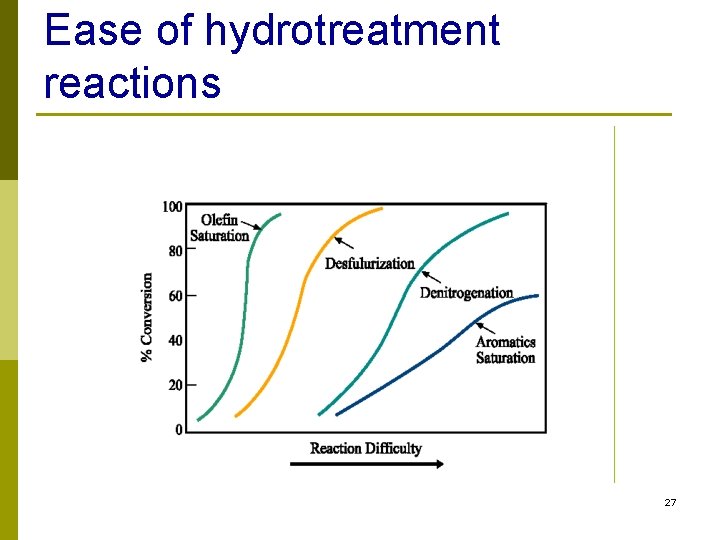
Increasing Difficulty Desulfurization 15

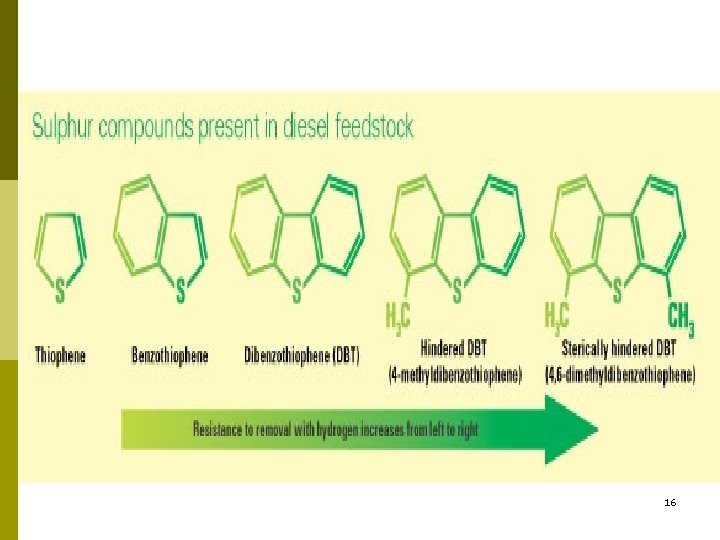
16

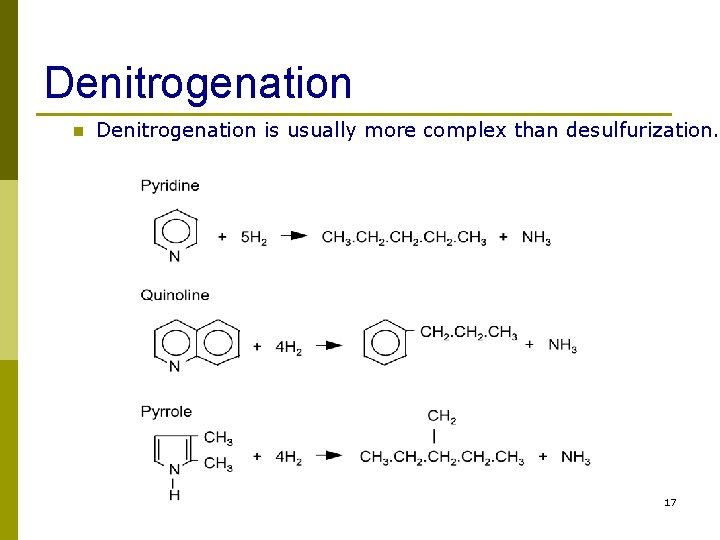
Denitrogenation n Denitrogenation is usually more complex than desulfurization. 17

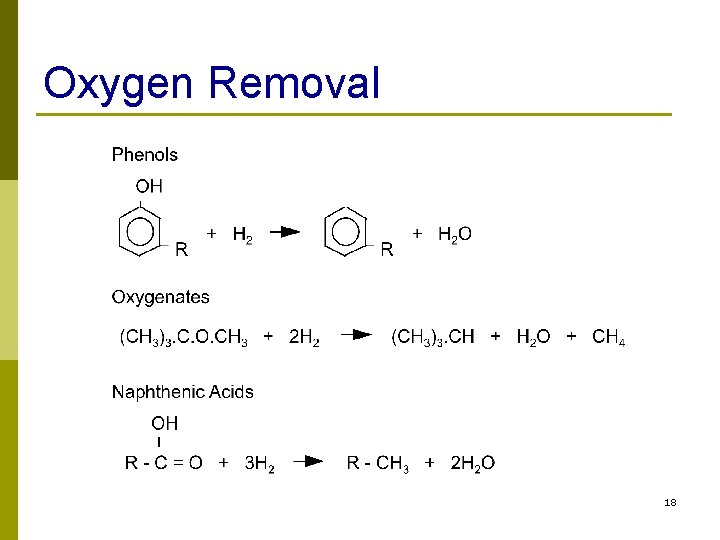
Oxygen Removal 18

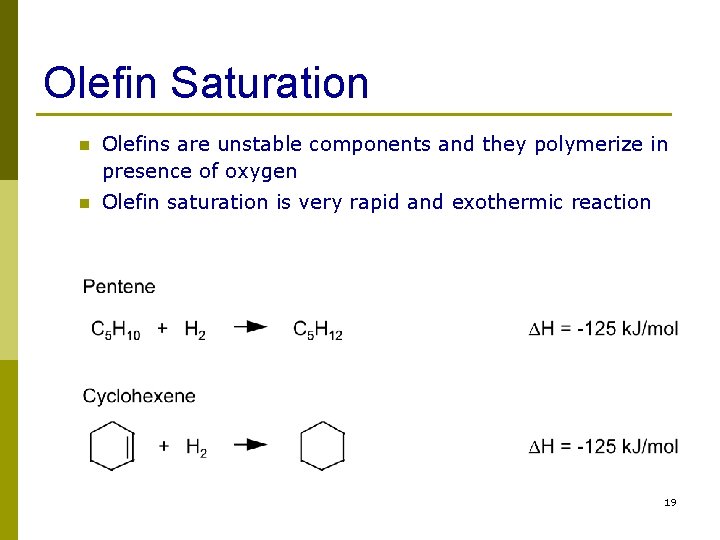
Olefin Saturation n Olefins are unstable components and they polymerize in presence of oxygen n Olefin saturation is very rapid and exothermic reaction 19

Aromatic Saturation p Aromatics present as 1, 2, 3 or more rings structure p Aromatic saturation is a stepwise reaction p Aromatic saturation is an exothermic reaction 20

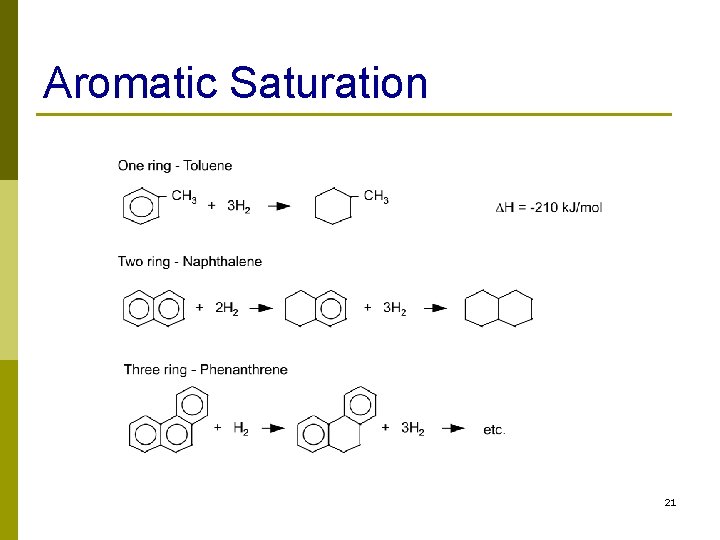
Aromatic Saturation 21

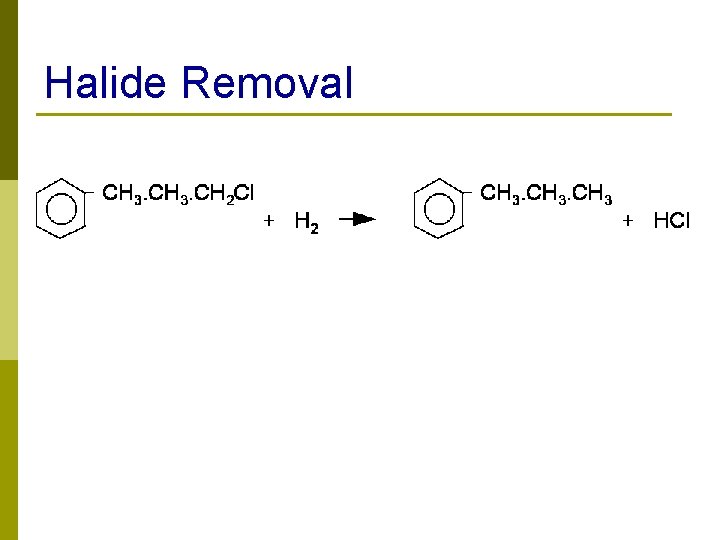
Halide Removal 22

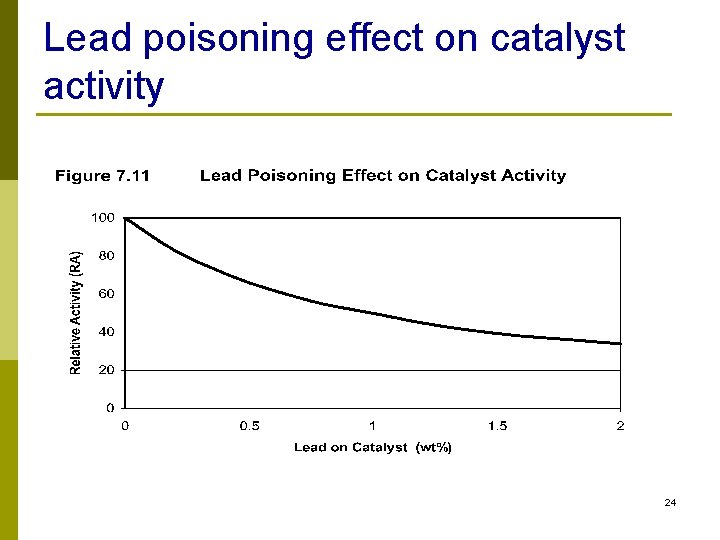
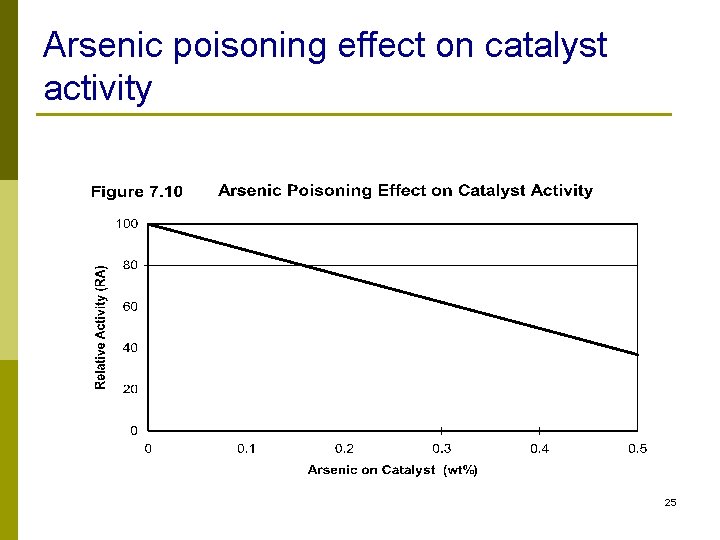
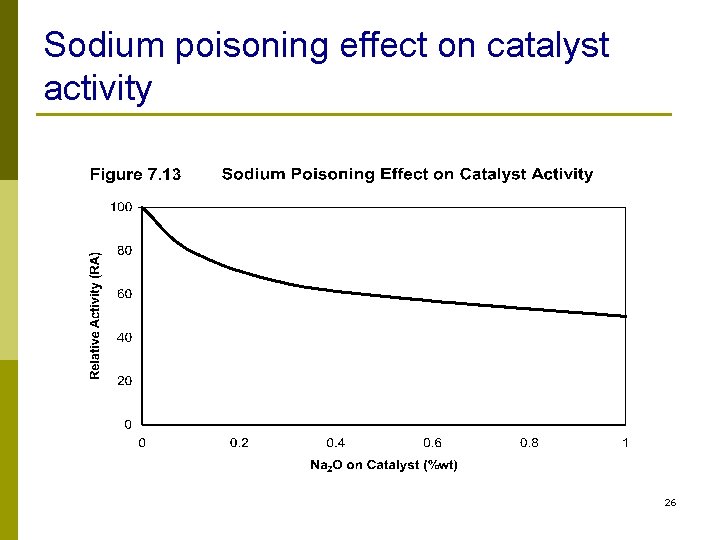
Metals Removal n Lead, mercury, arsenic, silicon, nickel, vanadium, sodium n Compounds that contain metals decompose in reactor and deactivate catalyst n The metal deposited on catalyst cannot be removed by regeneration 23

Lead poisoning effect on catalyst activity 24

Arsenic poisoning effect on catalyst activity 25

Sodium poisoning effect on catalyst activity 26

Ease of hydrotreatment reactions 27

28

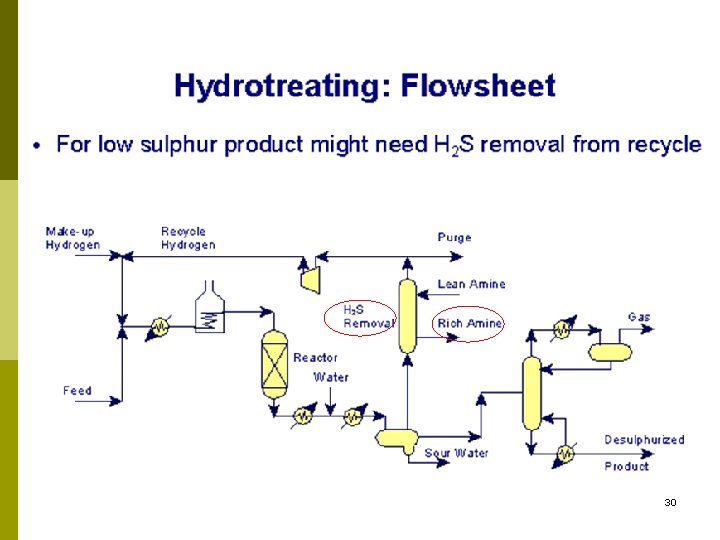
29

30

31

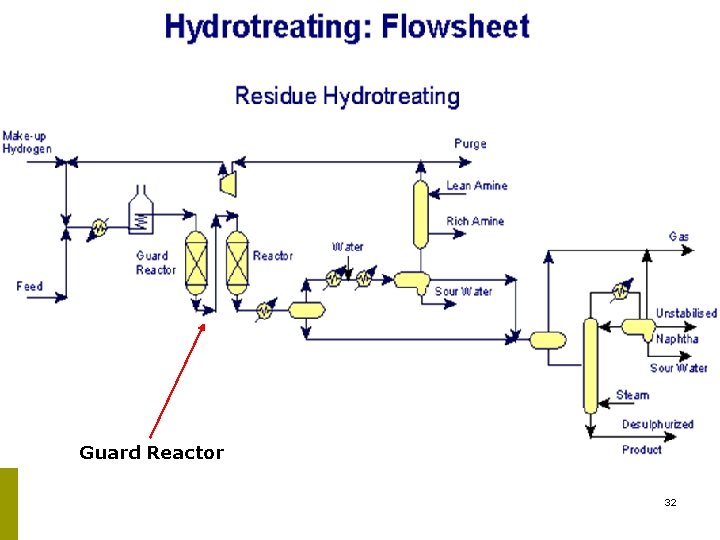
Guard Reactor 32

Hydrotreatment Catalyst Solid consisting of a base alumina impregnated with metal oxides p Pellets shaped as cylindrical or pills p For Desulfurization, Co-Mb catalyst is used p For Desulfurization, Denitrogenation and Aromatic saturation, Ni-Mb catalyst is used p 33

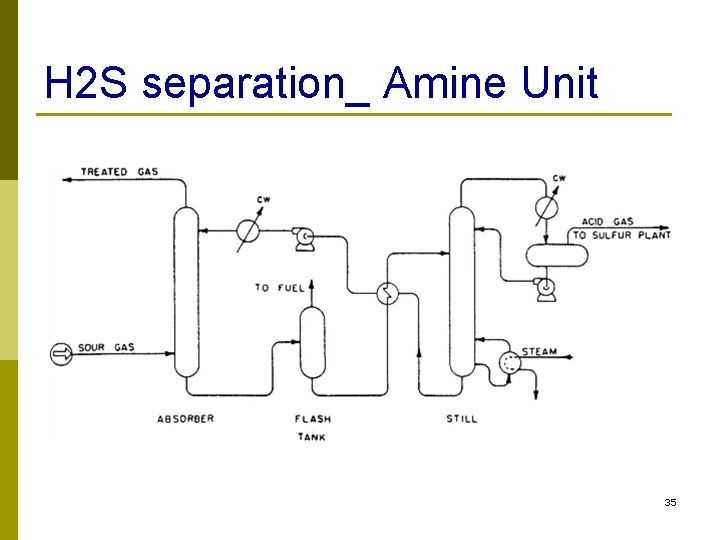
Sulfur recovery p Hydrogen sulfide created from hydrotreating is a toxic gas that needs further treatment p Solvent extraction: using diethanolamine (DEA) dissolved in water separates the H 2 S gas from the process stream (DEA should be recovered) 34

H 2 S separation_ Amine Unit 35

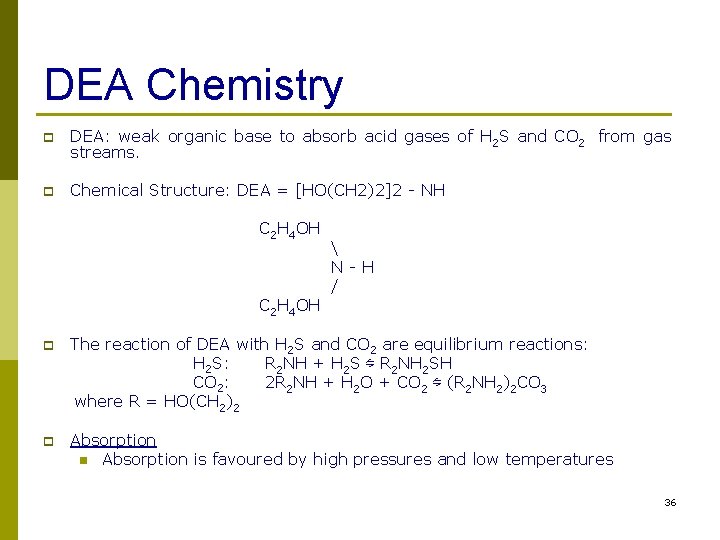
DEA Chemistry p DEA: weak organic base to absorb acid gases of H 2 S and CO 2 from gas streams. p Chemical Structure: DEA = [HO(CH 2)2]2 - NH C 2 H 4 OH N-H / p The reaction of DEA with H 2 S and CO 2 are equilibrium reactions: H 2 S: R 2 NH + H 2 S ⇋ R 2 NH 2 SH CO 2: 2 R 2 NH + H 2 O + CO 2 ⇋ (R 2 NH 2)2 CO 3 where R = HO(CH 2)2 p Absorption n Absorption is favoured by high pressures and low temperatures 36

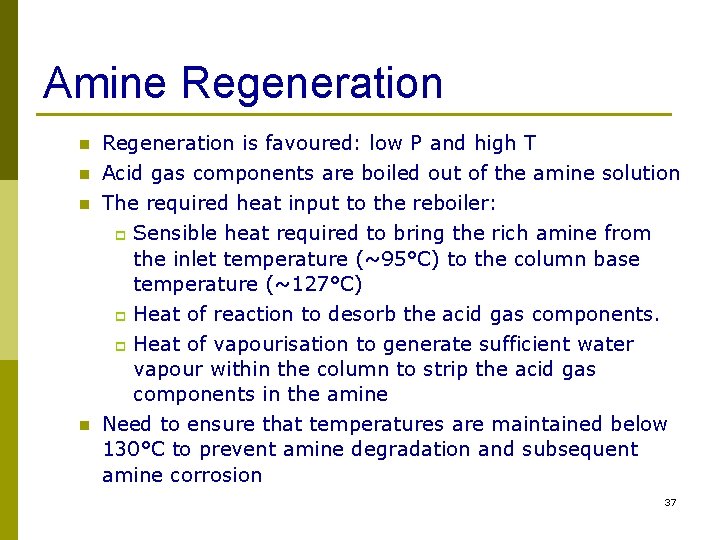
Amine Regeneration n Regeneration is favoured: low P and high T Acid gas components are boiled out of the amine solution The required heat input to the reboiler: p Sensible heat required to bring the rich amine from the inlet temperature (~95°C) to the column base temperature (~127°C) Heat of reaction to desorb the acid gas components. p Heat of vapourisation to generate sufficient water vapour within the column to strip the acid gas components in the amine Need to ensure that temperatures are maintained below 130°C to prevent amine degradation and subsequent amine corrosion p n 37

DEA Properties p Amine Strength: n n n p Ideally 25 -27 wt% DEA Higher amine concentrations = higher regenerator boiling temperatures and increased corrosion rates Lower amine concentrations = higher circulation rates and thus increase energy requirements for pumping and regeneration Amine Loading: n n Amine Loading = mol (H 2 S) + mol (CO 2) mol (DEA) Target is an amine loading of 0. 3 – 0. 5 mol/mol 38

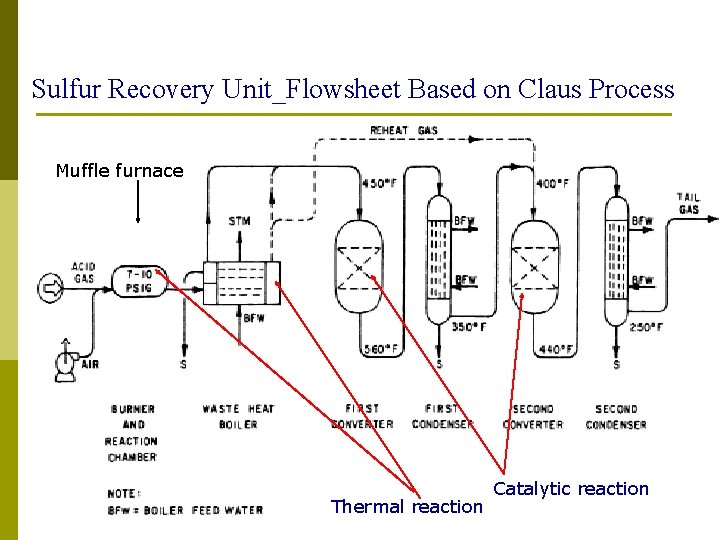
Sulfur Recovery Unit_Flowsheet Based on Claus Process Muffle furnace Thermal reaction Catalytic reaction

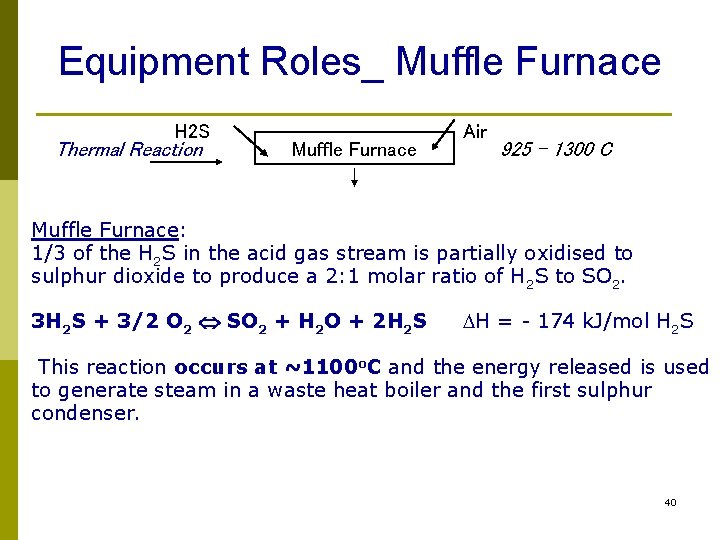
Equipment Roles_ Muffle Furnace H 2 S Thermal Reaction Muffle Furnace Air 925 – 1300 C Muffle Furnace: 1/3 of the H 2 S in the acid gas stream is partially oxidised to sulphur dioxide to produce a 2: 1 molar ratio of H 2 S to SO 2. 3 H 2 S + 3/2 O 2 SO 2 + H 2 O + 2 H 2 S DH = - 174 k. J/mol H 2 S This reaction occurs at ~1100 o. C and the energy released is used to generate steam in a waste heat boiler and the first sulphur condenser. 40

COS and CS 2 p Formation n The presence of CO 2 and HC in the feed gases to the Claus plant gives rise to carbonyl sulphide (COS) and carbon disulphide (CS 2) in the Muffle Furnace and Waste Heat Boiler n If these compounds are not hydrolysed back to H 2 S they will proceed through the catalytic reactors unreacted n This results in reduced sulphur conversion and decreased SRU efficiency. 41

COS COSand and. CS CS 2 2 p Destruction n The hydrolysis reactions can be written as: COS + H 2 O CO 2 + H 2 S CS 2 + 2 H 2 O CO 2 + 2 H 2 S n Hydrolysis is favoured by high temperatures (>310 C) n The ability of the catalyst to carry out the COS/CS 2 hydrolysis reactions depends on: 1. the catalyst formulation 2. the operating temperatures in the first reactor 3. the state of deactivation of the catalyst 42

Equipment Roles_ Waste Heat Boiler HP Steam – 3. 4 MPa Waste Heat Boiler: 1. Cools process stream 2. Recovers energy in a useful form (HP Steam) for use in the preheaters 3. Reactions also take place in the WHB 43

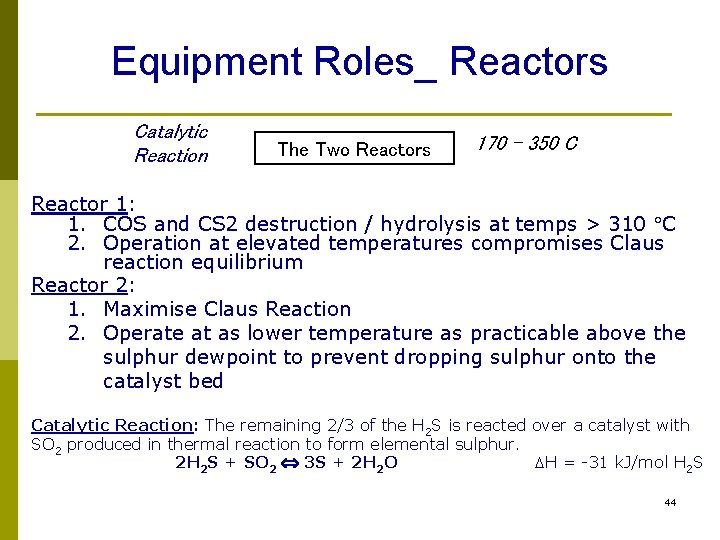
Equipment Roles_ Reactors Catalytic Reaction The Two Reactors 170 – 350 C Reactor 1: 1. COS and CS 2 destruction / hydrolysis at temps > 310 C 2. Operation at elevated temperatures compromises Claus reaction equilibrium Reactor 2: 1. Maximise Claus Reaction 2. Operate at as lower temperature as practicable above the sulphur dewpoint to prevent dropping sulphur onto the catalyst bed Catalytic Reaction: The remaining 2/3 of the H 2 S is reacted over a catalyst with SO 2 produced in thermal reaction to form elemental sulphur. 2 H 2 S + SO 2 3 S + 2 H 2 O DH = -31 k. J/mol H 2 S 44

Equipment Roles_ Condensers Sulphur Condensers LP Steam – 345 k. Pa Liquid Sulphur Condensers: Recover produced sulphur 45

Equipment Roles_ Incinerator Incineration or Further Treatment Incinerator: Combust unreacted H 2 S to SO 2 prior to emission of tail gas to the environment 46

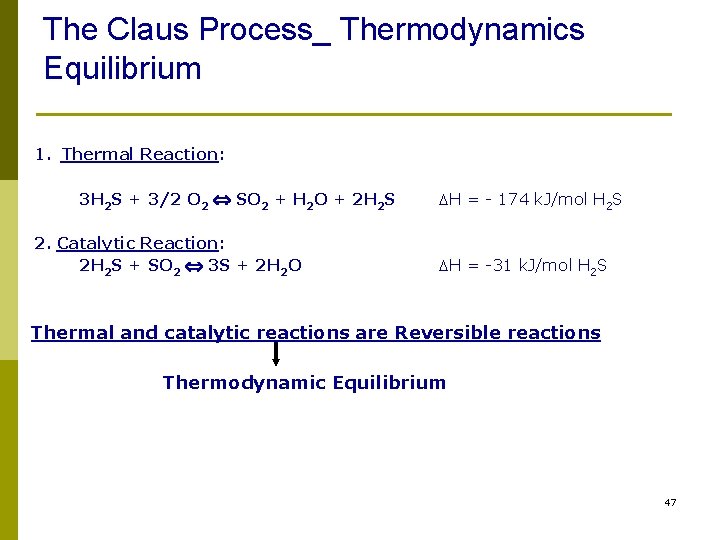
The Claus Process_ Thermodynamics Equilibrium p 1. Thermal Reaction: 3 H 2 S + 3/2 O 2 SO 2 + H 2 O + 2 H 2 S 2. Catalytic Reaction: 2 H 2 S + SO 2 3 S + 2 H 2 O DH = - 174 k. J/mol H 2 S DH = -31 k. J/mol H 2 S Thermal and catalytic reactions are Reversible reactions Thermodynamic Equilibrium 47

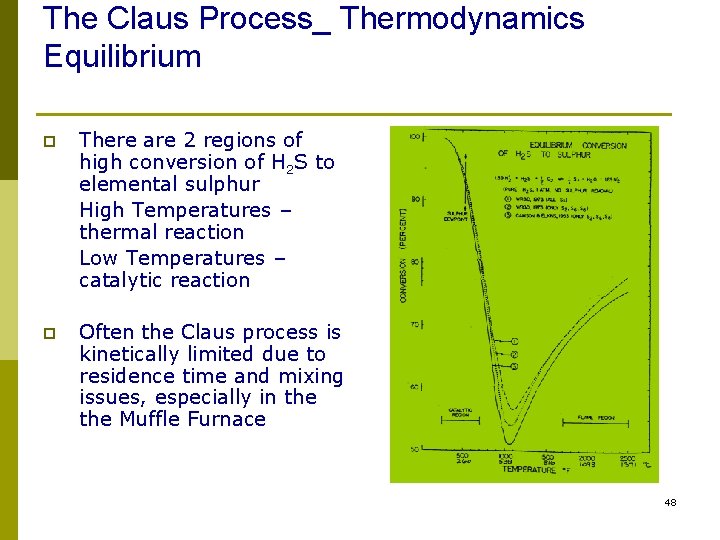
The Claus Process_ Thermodynamics Equilibrium p There are 2 regions of high conversion of H 2 S to elemental sulphur High Temperatures – thermal reaction Low Temperatures – catalytic reaction p Often the Claus process is kinetically limited due to residence time and mixing issues, especially in the Muffle Furnace 48

SRU Catalyst p Standard Catalyst n n n Activated alumina (Al 2 O 3) – (Co-Mb) Average life in a refinery SRU is 3 -5 years Important catalyst properties p p Surface area: 320 – 390 m 2/g Pore volume distribution: to ensure bulk diffusion to internal surface area Physical and mechanical properties: crush strength Promoted Catalyst n n Titanium dioxide (Ti. O 2) COS / CS 2 hydrolysis, O 2 scavenger, deactivation resistance Poor crush strength Expensive 49

SRU Efficiency p Sulphur recovery efficiency is a measure of the percentage of sulphur present in SRU feed as H 2 S, that is converted and recovered as liquid elemental sulphur. p SRU recovery efficiency is: n n dependent upon all significant operating conditions a direct measure of sulphur plant performance is independent of plant size can be measured accurately 50

SRU Efficiency p It can be represented by the following equation: Recovery = SPRODUCED/SFEED(as H 2 S) p The efficiency of a conventional Claus plant is limited by: n the efficiency of COS/CS 2 hydrolysis in the first catalytic reactor n the equilibrium conversion of H 2 S and SO 2 to elemental sulphur at the minimum safe operating temperature n the saturation vapour pressure of sulphur at the minimum safe condenser operating temperature. 51

To Maximise SRU Efficiency p Minimise impurity level in the feed n n n Impurities all reduce the partial pressures of the Claus Reactants CO 2 acts as a ‘fire extinguisher’ in the Muffle Furnace decreasing temperatures, decreases plant capacity and leads to the production of COS Natural Gas/HC leads to the production of CS 2, increases air demand, and cause possible downstream contamination H 2 O is a product of the Claus Reaction NH 3 increases air demand, and can lead to plugging from ammonium salts 52

To Maximise SRU Efficiency p Maximise product removal at earliest opportunity p Use 3 catalytic stages with active catalyst Maximise Reactor 1 temperatures to maximise COS and CS 2 destruction p p Optimal air control n H 2 S and O 2 should be delivered to the muffle furnace in the correct stoichiometric ratio n Excess O 2 can result in sulphation of the catalyst = deactivation 53

End of Chapter Nine 54