CRUDE OIL A guide for GCSE students KNOCKHARDY
















- Slides: 54

CRUDE OIL A guide for GCSE students KNOCKHARDY PUBLISHING

CRUDE OIL INTRODUCTION This Powerpoint show is one of several produced to help students understand selected GCSE Chemistry topics. It is based on the requirements of the AQA specification but is suitable for other examination boards. Individual students may use the material at home for revision purposes and it can also prove useful for classroom teaching with an interactive white board. Accompanying notes on this, and the full range of AS and A 2 Chemistry topics, are available from the KNOCKHARDY WEBSITE at. . . www. knockhardy. org. uk All diagrams, photographs and any animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any work that is distributed for financial gain.

CRUDE OIL CONTENTS • Origin of crude oil • Fractional distillation • Cracking For more detailed information on the properties of hydrocarbons such as alkanes and alkenes, see the appropriate Powerpoint on the Knockhardy GCSE site. www. knockhardy. org. uk/gcse. htm

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE A mixture consists of two or more elements or compounds not chemically combined together. Other examples AIR SMARTIES contains different gases contains different sweets

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged The oxygen in air behaves as oxygen The orange Smarties® in SMARTIES® taste of orange

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods including filtration, chromatography, distillation and, for crude oil, fractional distillation.

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE CONTAINS A VERY LARGE NUMBER OF COMPOUNDS

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE CONTAINS A VERY LARGE NUMBER OF COMPOUNDS CONTAINS MAINLY HYDROCARBONS (compounds containing only carbon and hydrogen)

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE CONTAINS A VERY LARGE NUMBER OF COMPOUNDS CONTAINS MAINLY HYDROCARBONS ALSO CONTAINS SULPHUR + SULPHUR COMPOUNDS IS A DARK COLOURED VISCOUS LIQUID

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE CONTAINS A VERY LARGE NUMBER OF COMPOUNDS CONTAINS MAINLY HYDROCARBONS ALSO CONTAINS SULPHUR + SULPHUR COMPOUNDS IS A DARK COLOURED VISCOUS LIQUID

CRUDE OIL WHAT IS IT? CRUDE OIL IS A FOSSIL FUEL DERIVED FROM AN ANCIENT BIOMASS FOUND IN ROCKS IS A MIXTURE CONTAINS A VERY LARGE NUMBER OF COMPOUNDS CONTAINS MAINLY HYDROCARBONS ALSO CONTAINS SULPHUR + SULPHUR COMPOUNDS IS A DARK COLOURED VISCOUS LIQUID

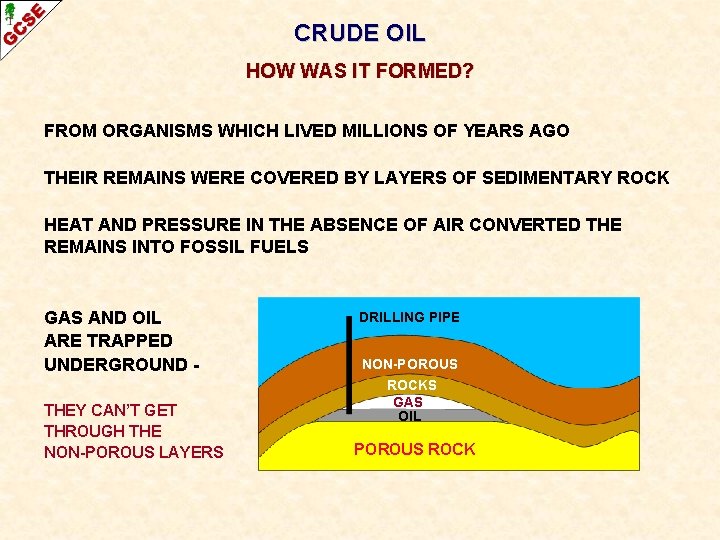
CRUDE OIL HOW WAS IT FORMED? FROM ORGANISMS WHICH LIVED MILLIONS OF YEARS AGO THEIR REMAINS WERE COVERED BY LAYERS OF SEDIMENTARY ROCK HEAT AND PRESSURE IN THE ABSENCE OF AIR CONVERTED THE REMAINS INTO FOSSIL FUELS GAS AND OIL ARE TRAPPED UNDERGROUND THEY CAN’T GET THROUGH THE NON-POROUS LAYERS DRILLING PIPE NON-POROUS ROCKS GAS OIL POROUS ROCK

CRUDE OIL HOW IS IT PROCESSED? CRUDE OIL CAN BE SPLIT INTO FRACTIONS - GROUPS OF COMPOUNDS WITH SIMILAR BOILING POINTS THE PROCESS IS CALLED FRACTIONAL DISTILLATION

FRACTIONAL DISTILLATION

FRACTIONAL DISTILLATION WHAT HAPPENS? THE DISTILLATION TOWER

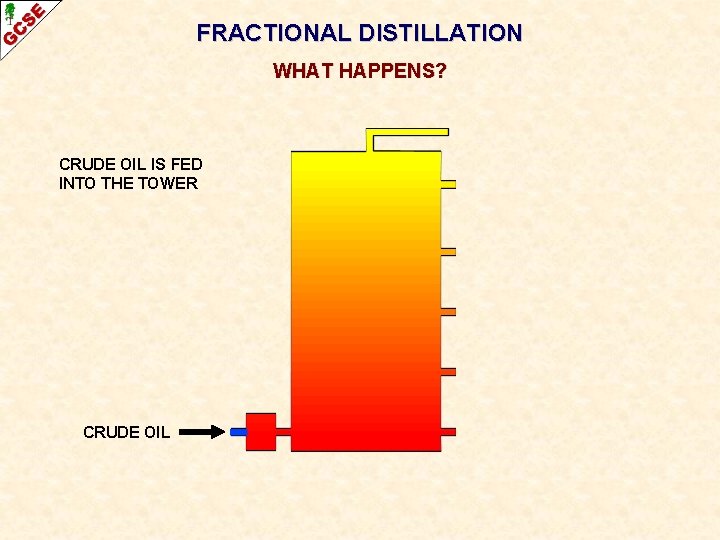
FRACTIONAL DISTILLATION WHAT HAPPENS? CRUDE OIL IS FED INTO THE TOWER CRUDE OIL

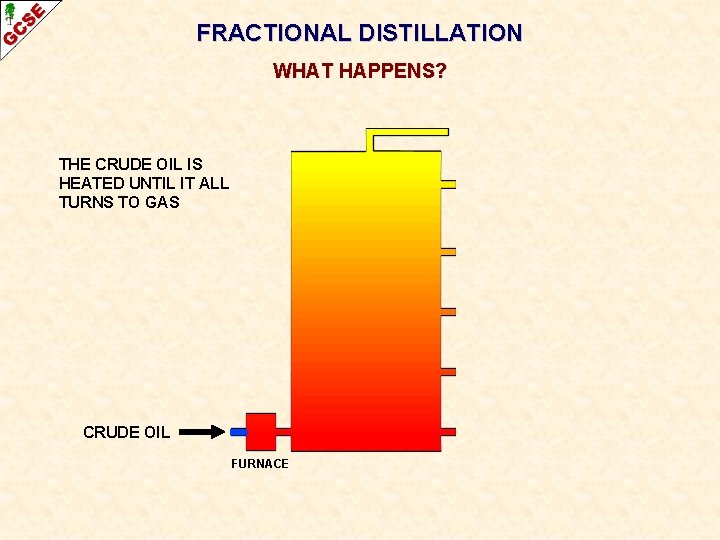
FRACTIONAL DISTILLATION WHAT HAPPENS? THE CRUDE OIL IS HEATED UNTIL IT ALL TURNS TO GAS CRUDE OIL FURNACE

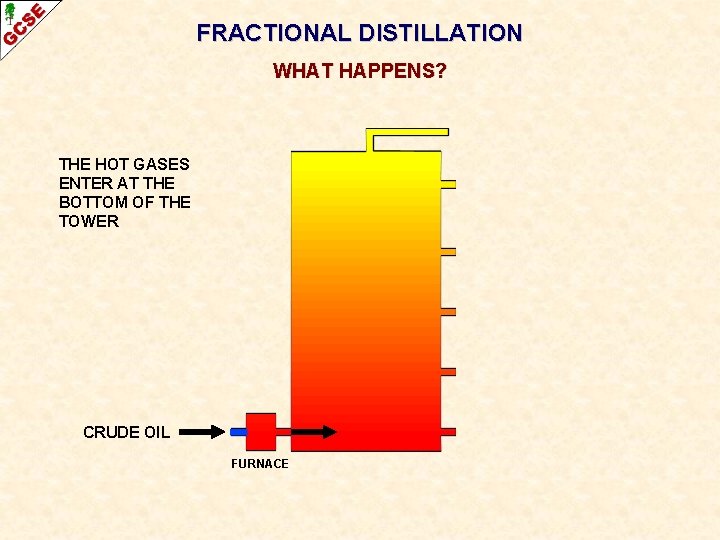
FRACTIONAL DISTILLATION WHAT HAPPENS? THE HOT GASES ENTER AT THE BOTTOM OF THE TOWER CRUDE OIL FURNACE

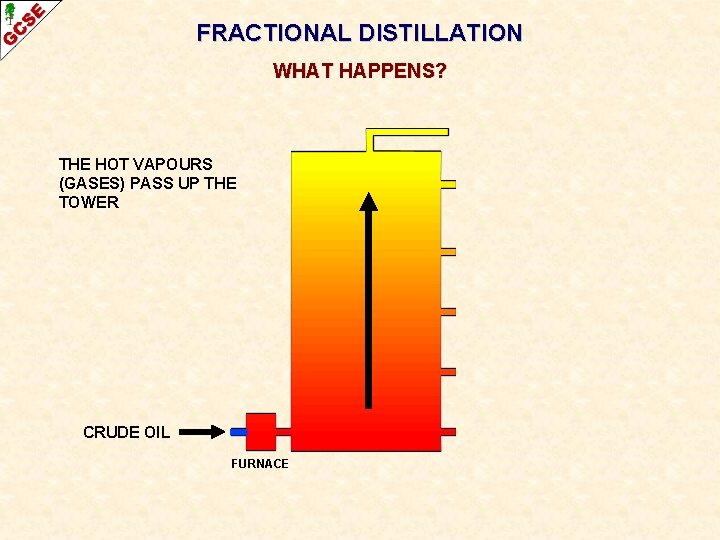
FRACTIONAL DISTILLATION WHAT HAPPENS? THE HOT VAPOURS (GASES) PASS UP THE TOWER CRUDE OIL FURNACE

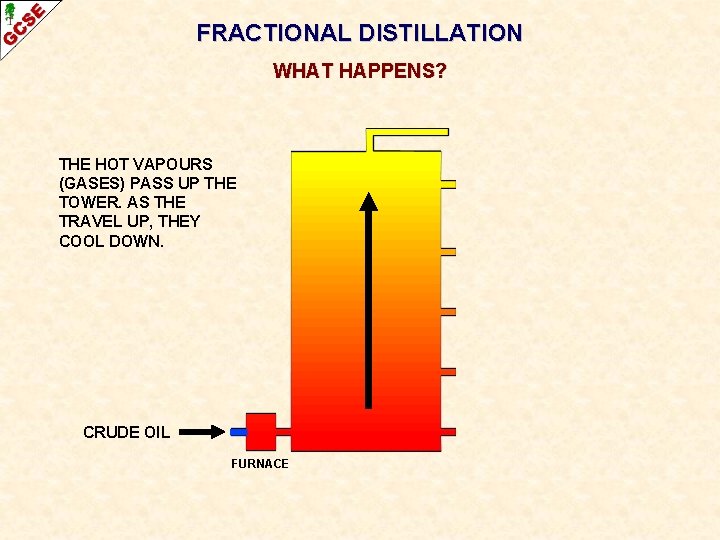
FRACTIONAL DISTILLATION WHAT HAPPENS? THE HOT VAPOURS (GASES) PASS UP THE TOWER. AS THE TRAVEL UP, THEY COOL DOWN. CRUDE OIL FURNACE

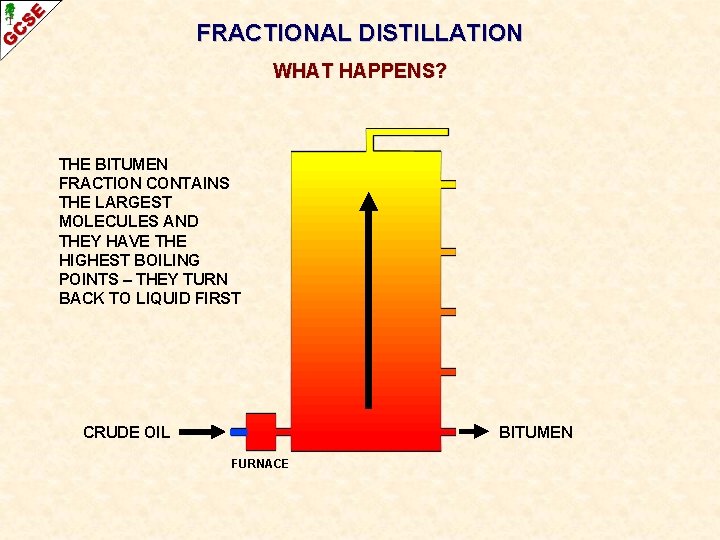
FRACTIONAL DISTILLATION WHAT HAPPENS? THE BITUMEN FRACTION CONTAINS THE LARGEST MOLECULES AND THEY HAVE THE HIGHEST BOILING POINTS – THEY TURN BACK TO LIQUID FIRST CRUDE OIL BITUMEN FURNACE

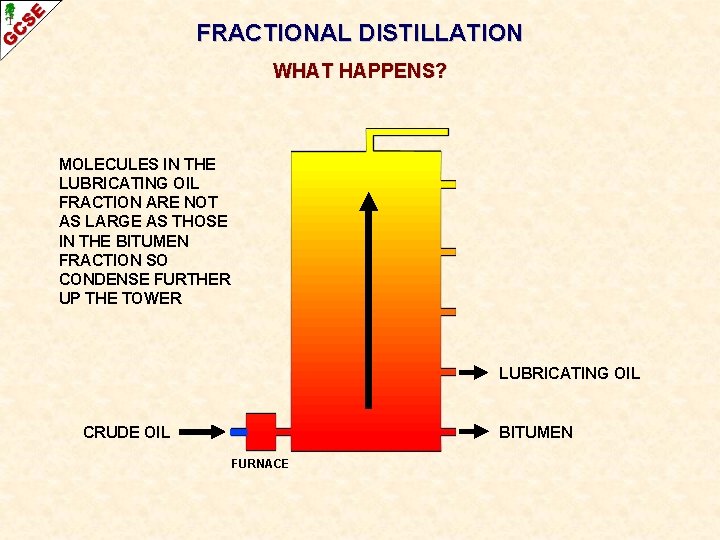
FRACTIONAL DISTILLATION WHAT HAPPENS? MOLECULES IN THE LUBRICATING OIL FRACTION ARE NOT AS LARGE AS THOSE IN THE BITUMEN FRACTION SO CONDENSE FURTHER UP THE TOWER LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

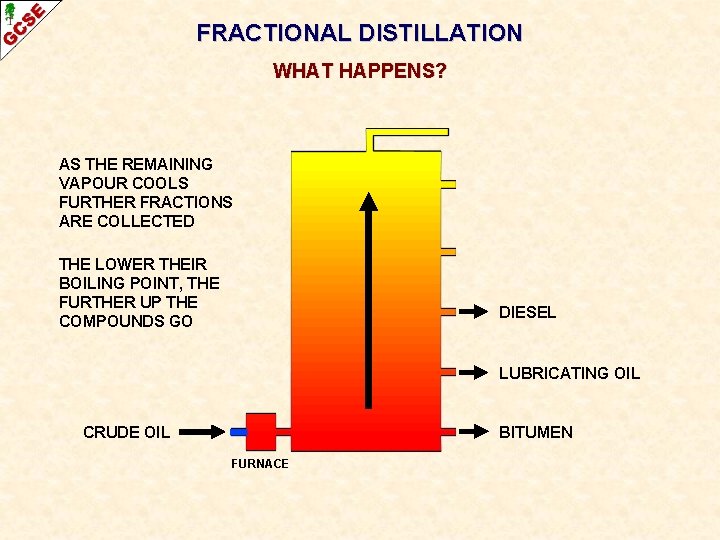
FRACTIONAL DISTILLATION WHAT HAPPENS? AS THE REMAINING VAPOUR COOLS FURTHER FRACTIONS ARE COLLECTED THE LOWER THEIR BOILING POINT, THE FURTHER UP THE COMPOUNDS GO DIESEL LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

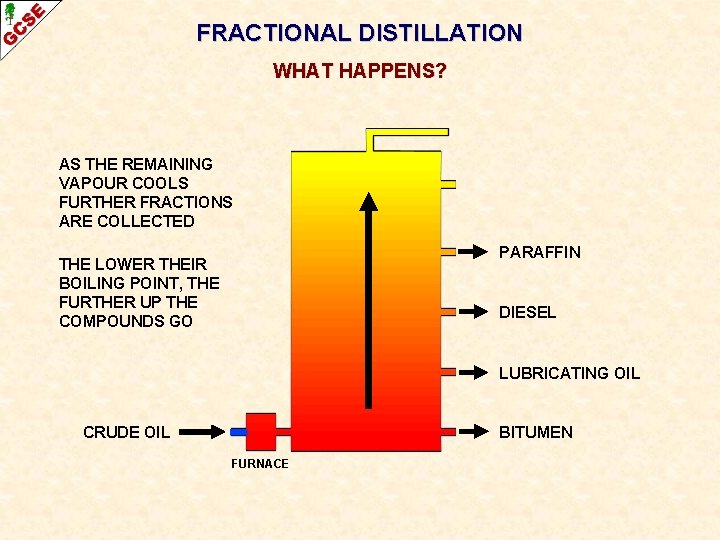
FRACTIONAL DISTILLATION WHAT HAPPENS? AS THE REMAINING VAPOUR COOLS FURTHER FRACTIONS ARE COLLECTED PARAFFIN THE LOWER THEIR BOILING POINT, THE FURTHER UP THE COMPOUNDS GO DIESEL LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

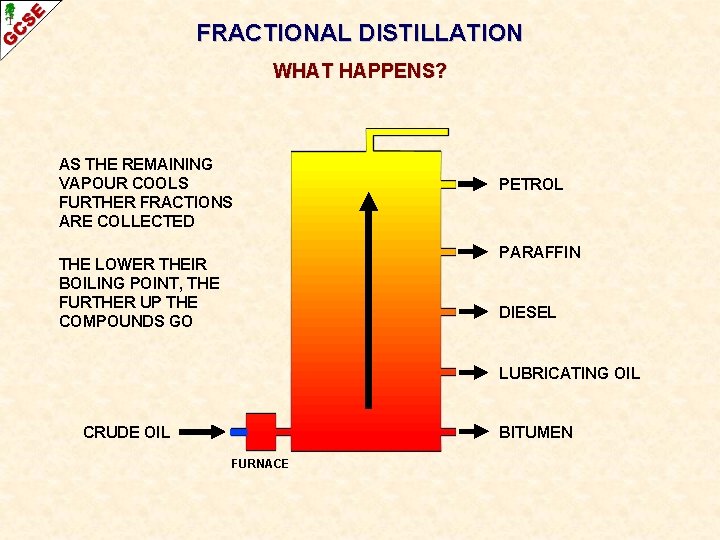
FRACTIONAL DISTILLATION WHAT HAPPENS? AS THE REMAINING VAPOUR COOLS FURTHER FRACTIONS ARE COLLECTED PETROL PARAFFIN THE LOWER THEIR BOILING POINT, THE FURTHER UP THE COMPOUNDS GO DIESEL LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

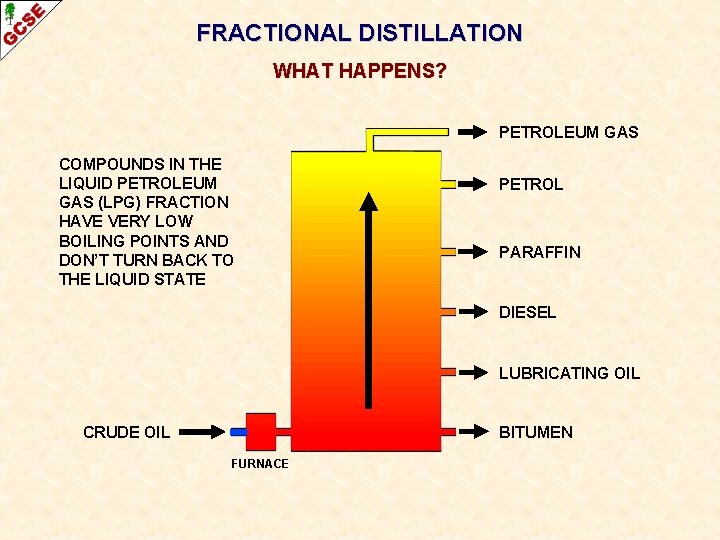
FRACTIONAL DISTILLATION WHAT HAPPENS? PETROLEUM GAS COMPOUNDS IN THE LIQUID PETROLEUM GAS (LPG) FRACTION HAVE VERY LOW BOILING POINTS AND DON’T TURN BACK TO THE LIQUID STATE PETROL PARAFFIN DIESEL LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

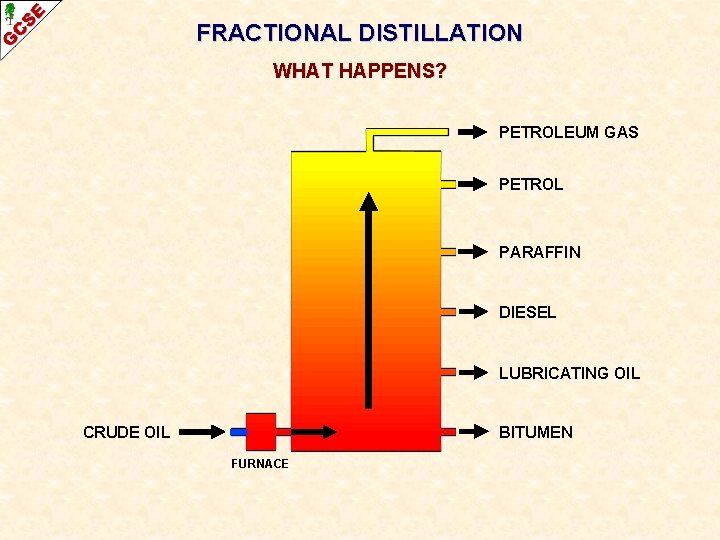
FRACTIONAL DISTILLATION WHAT HAPPENS? PETROLEUM GAS PETROL PARAFFIN DIESEL LUBRICATING OIL CRUDE OIL BITUMEN FURNACE

FRACTIONAL DISTILLATION ANIMATION

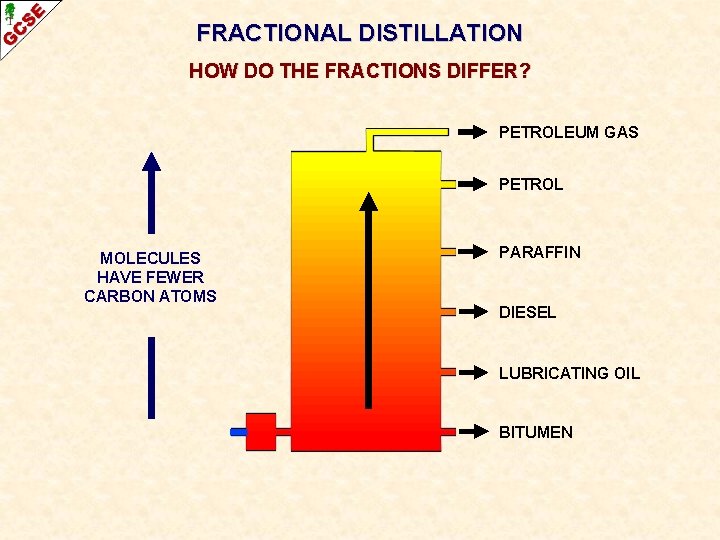
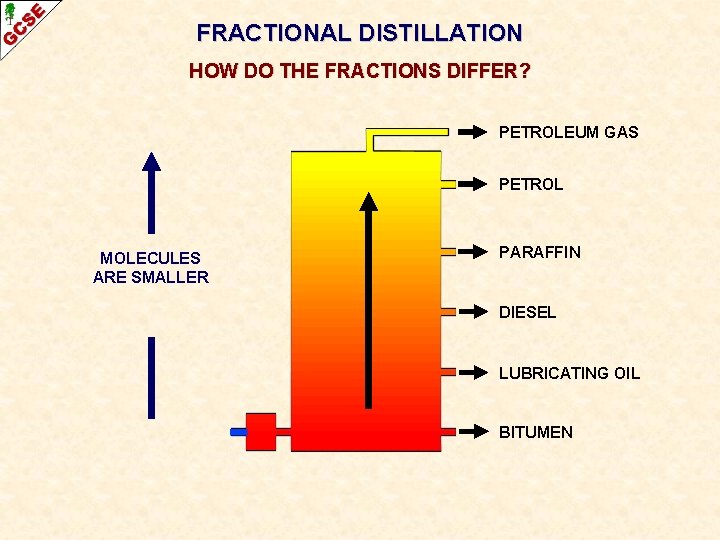
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES HAVE FEWER CARBON ATOMS PARAFFIN DIESEL LUBRICATING OIL BITUMEN

FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES ARE SMALLER PARAFFIN DIESEL LUBRICATING OIL BITUMEN

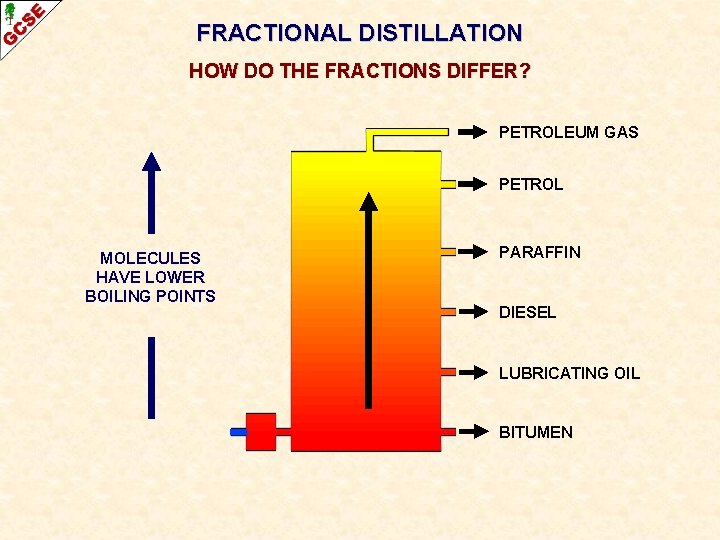
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES HAVE LOWER BOILING POINTS PARAFFIN DIESEL LUBRICATING OIL BITUMEN

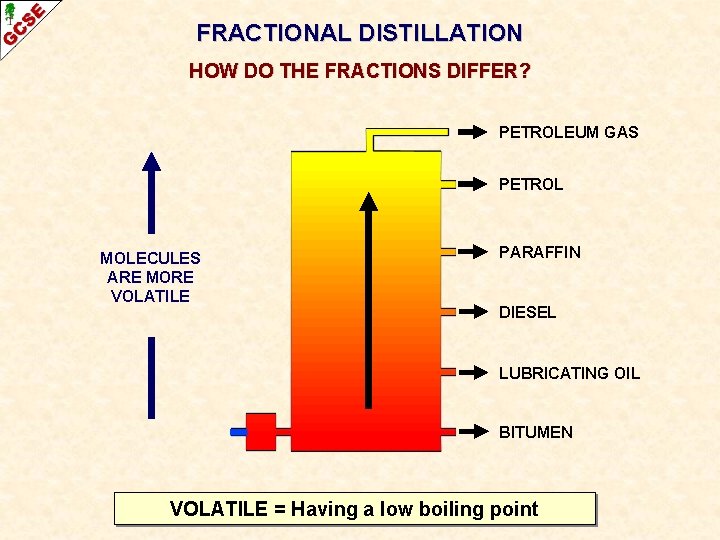
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES ARE MORE VOLATILE PARAFFIN DIESEL LUBRICATING OIL BITUMEN VOLATILE = Having a low boiling point

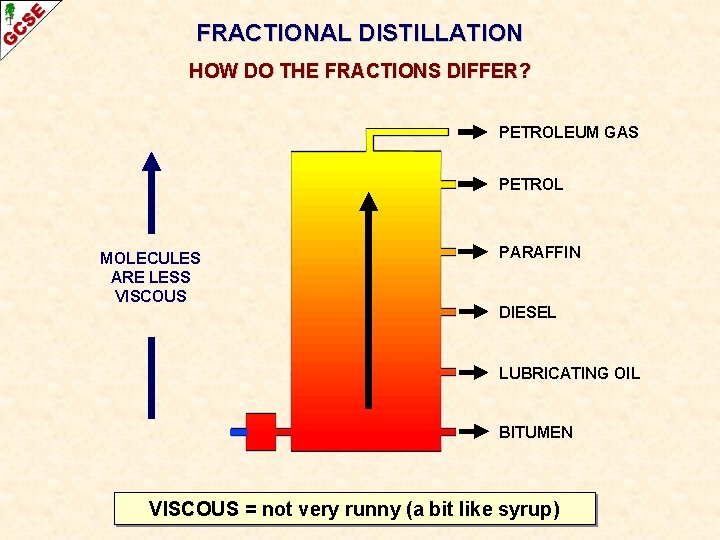
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES ARE LESS VISCOUS PARAFFIN DIESEL LUBRICATING OIL BITUMEN VISCOUS = not very runny (a bit like syrup)

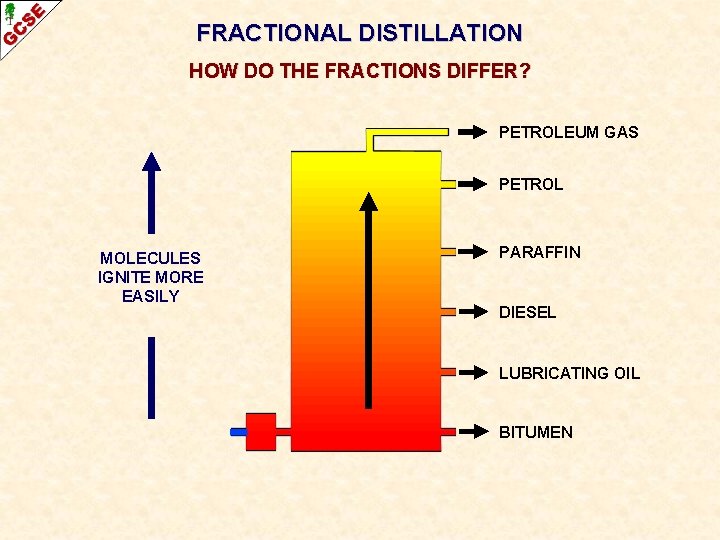
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES IGNITE MORE EASILY PARAFFIN DIESEL LUBRICATING OIL BITUMEN

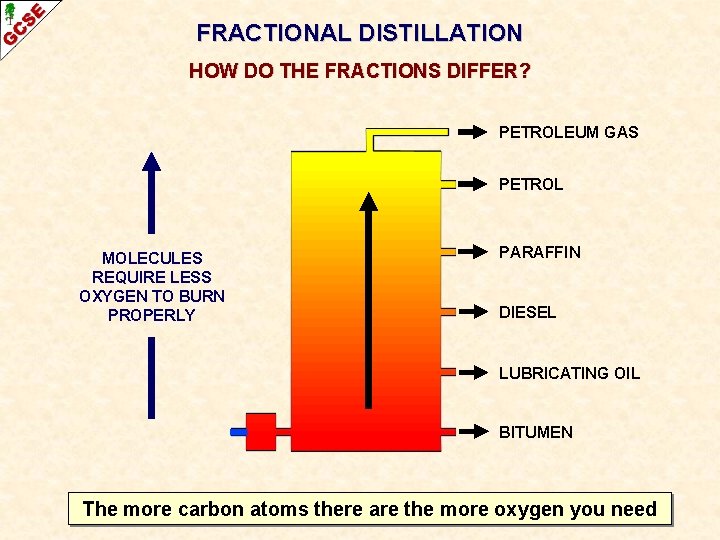
FRACTIONAL DISTILLATION HOW DO THE FRACTIONS DIFFER? PETROLEUM GAS PETROL MOLECULES REQUIRE LESS OXYGEN TO BURN PROPERLY PARAFFIN DIESEL LUBRICATING OIL BITUMEN The more carbon atoms there are the more oxygen you need

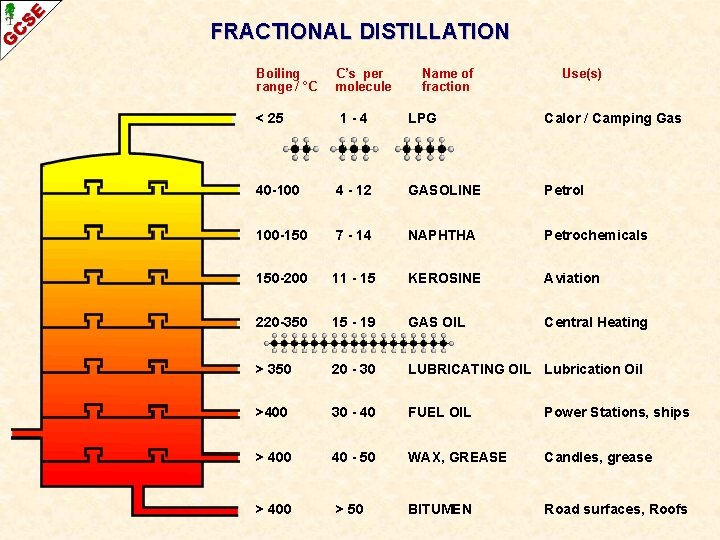
FRACTIONAL DISTILLATION Boiling range / °C C’s per molecule Name of fraction Use(s) < 25 1 -4 LPG Calor / Camping Gas 40 -100 4 - 12 GASOLINE Petrol 100 -150 7 - 14 NAPHTHA Petrochemicals 150 -200 11 - 15 KEROSINE Aviation 220 -350 15 - 19 GAS OIL Central Heating > 350 20 - 30 LUBRICATING OIL Lubrication Oil >400 30 - 40 FUEL OIL Power Stations, ships > 400 40 - 50 WAX, GREASE Candles, grease > 400 > 50 BITUMEN Road surfaces, Roofs

FRACTIONAL DISTILLATION WHAT HAPPENS TO THE FRACTIONS? FRACTIONS ARE STILL MIXTURES DIFFERENT FRACTIONS ARE PUT TO DIFFERENT USES FRACTIONS CAN BE DISTILLED FURTHER SOME FRACTIONS ARE MORE VALUABLE THAN OTHERS THE HEAVIER FRACTIONS ARE LESS VALUABLE

FRACTIONAL DISTILLATION WHAT HAPPENS TO THE FRACTIONS? FRACTIONS ARE STILL MIXTURES DIFFERENT FRACTIONS ARE PUT TO DIFFERENT USES FRACTIONS CAN BE DISTILLED FURTHER SOME FRACTIONS ARE MORE VALUABLE THAN OTHERS THE HEAVIER FRACTIONS ARE LESS VALUABLE LARGER MOLECULES CAN BE BROKEN DOWN TO SMALLER ONES - THIS IS KNOWN AS CRACKING

CRACKING

CRACKING WHY IS IT DONE? LARGER HYDROCARBON MOLECULES ARE LESS USEFUL THEY CAN BE BROKEN DOWN INTO SMALLER MOLECULES ARE MORE USEFUL

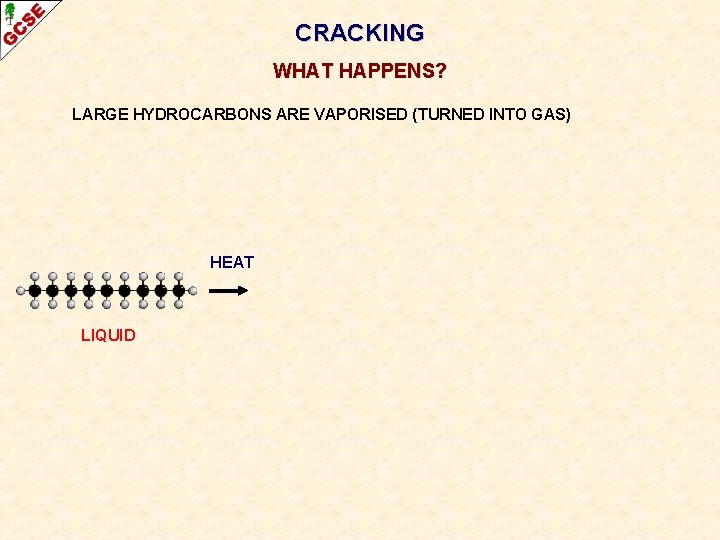
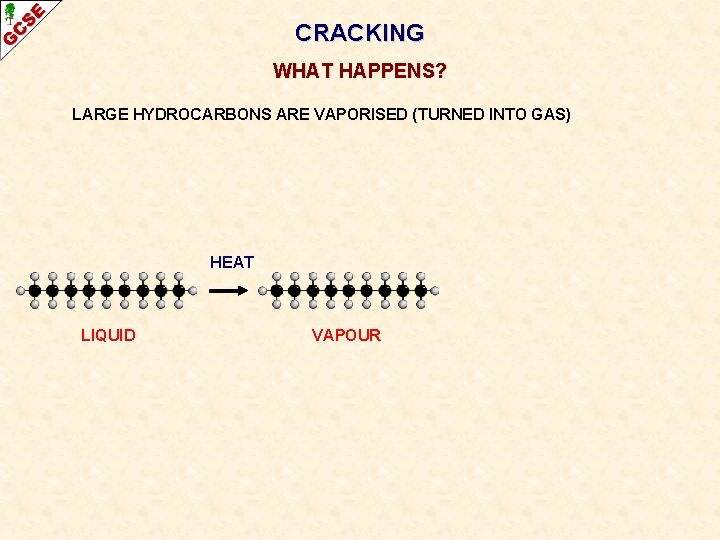
CRACKING WHAT HAPPENS? LARGE HYDROCARBONS ARE VAPORISED (TURNED INTO GAS) HEAT LIQUID

CRACKING WHAT HAPPENS? LARGE HYDROCARBONS ARE VAPORISED (TURNED INTO GAS) HEAT LIQUID VAPOUR

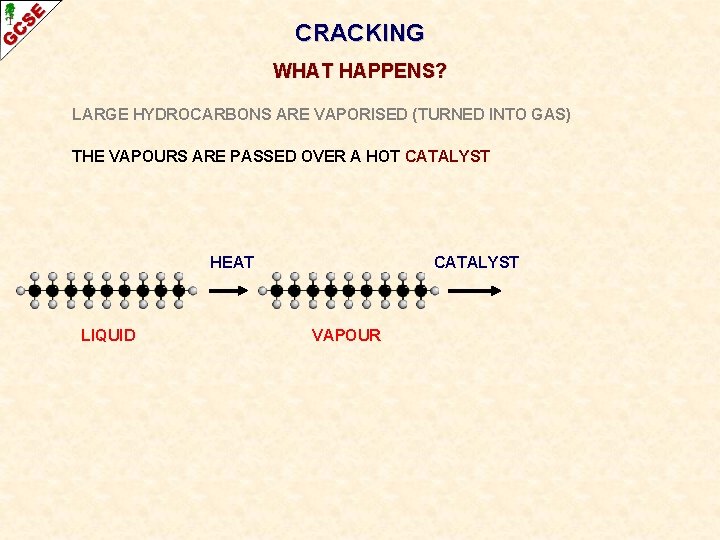
CRACKING WHAT HAPPENS? LARGE HYDROCARBONS ARE VAPORISED (TURNED INTO GAS) THE VAPOURS ARE PASSED OVER A HOT CATALYST HEAT LIQUID CATALYST VAPOUR

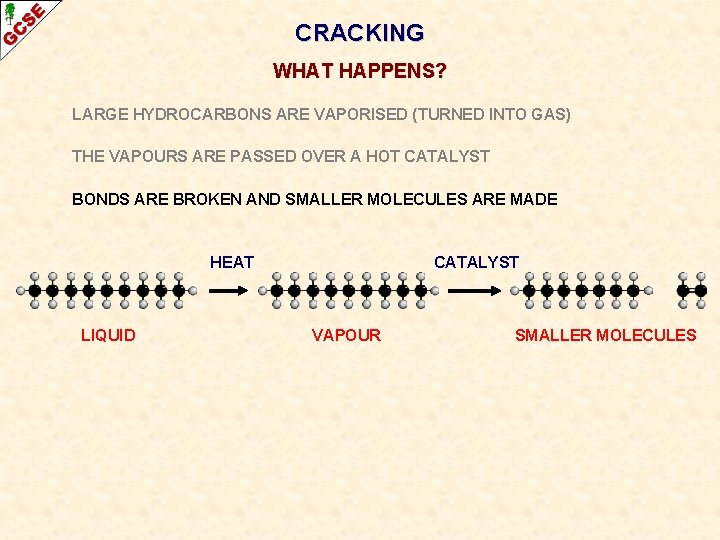
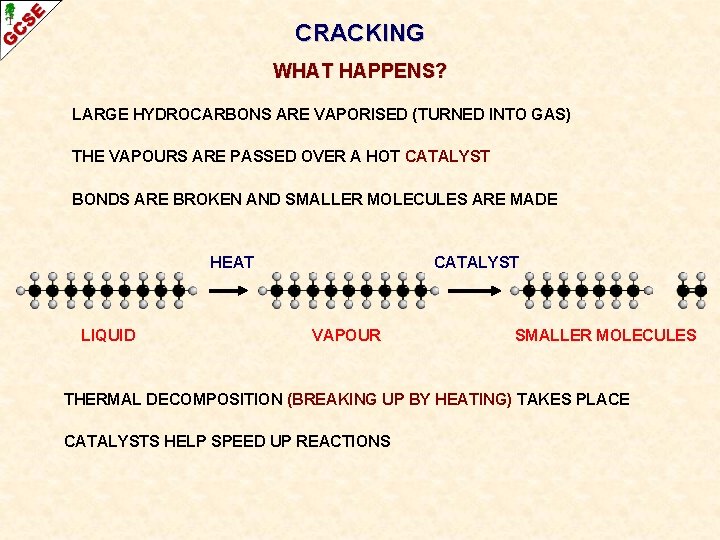
CRACKING WHAT HAPPENS? LARGE HYDROCARBONS ARE VAPORISED (TURNED INTO GAS) THE VAPOURS ARE PASSED OVER A HOT CATALYST BONDS ARE BROKEN AND SMALLER MOLECULES ARE MADE HEAT LIQUID CATALYST VAPOUR SMALLER MOLECULES

CRACKING WHAT HAPPENS? LARGE HYDROCARBONS ARE VAPORISED (TURNED INTO GAS) THE VAPOURS ARE PASSED OVER A HOT CATALYST BONDS ARE BROKEN AND SMALLER MOLECULES ARE MADE HEAT LIQUID CATALYST VAPOUR SMALLER MOLECULES THERMAL DECOMPOSITION (BREAKING UP BY HEATING) TAKES PLACE CATALYSTS HELP SPEED UP REACTIONS

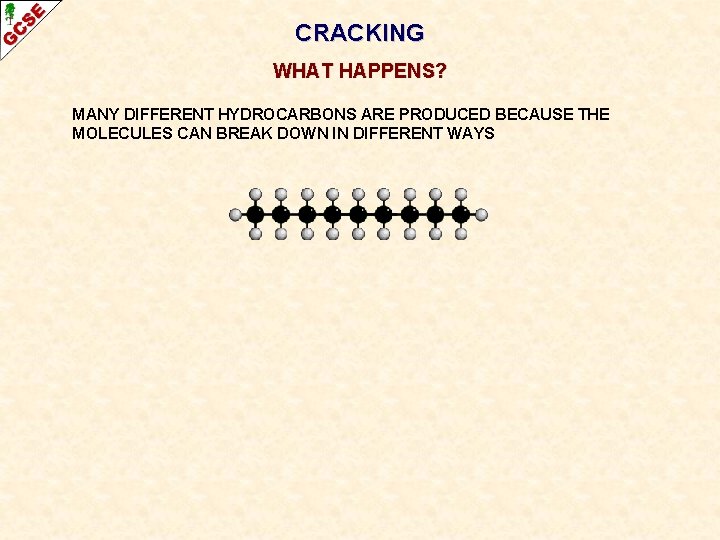
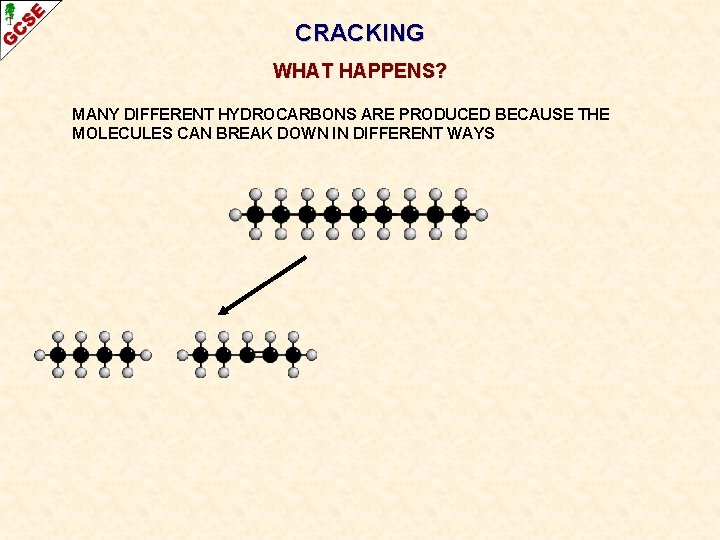
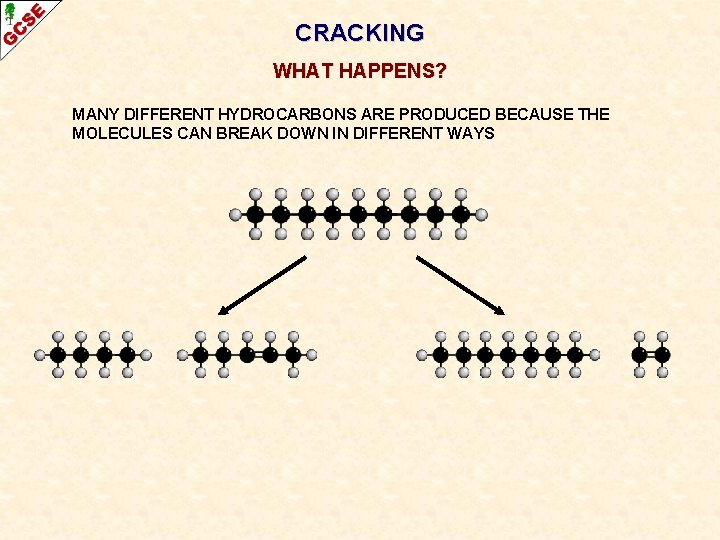
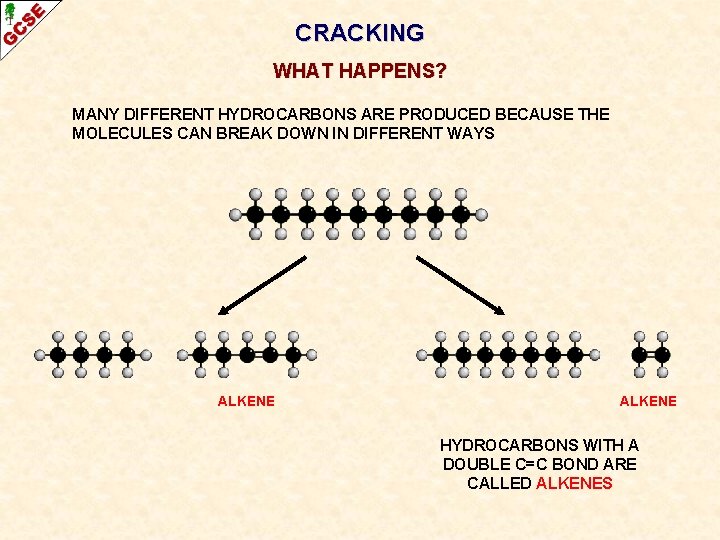
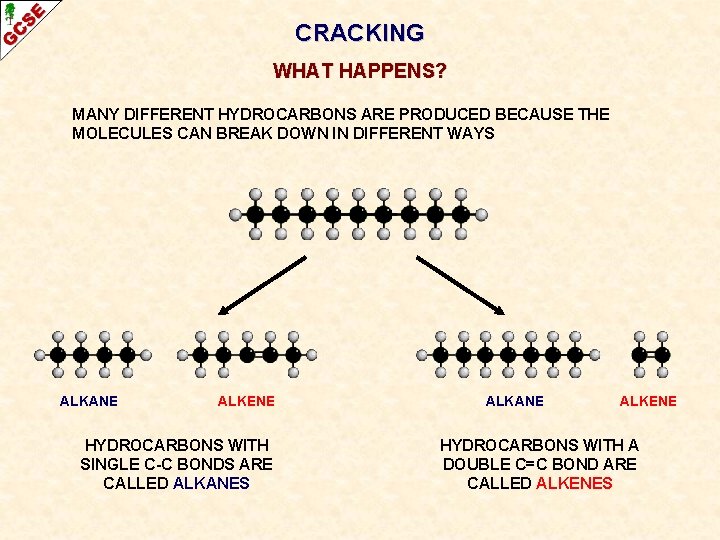
CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS

CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS

CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS

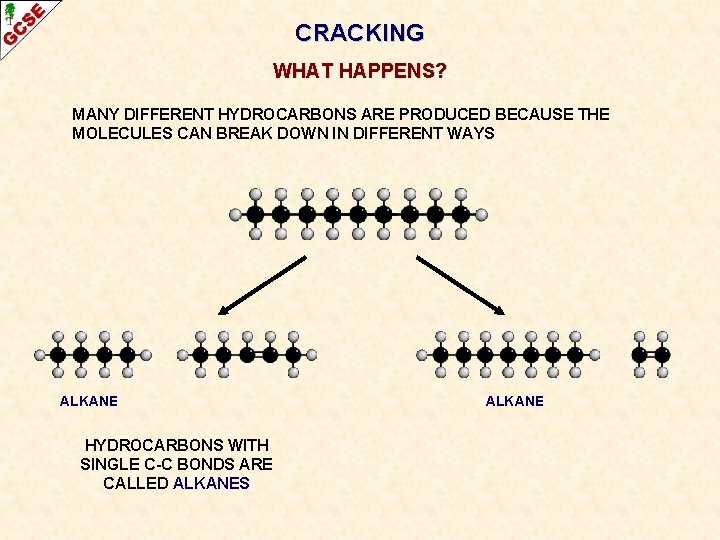
CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS ALKANE HYDROCARBONS WITH SINGLE C-C BONDS ARE CALLED ALKANES ALKANE

CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS ALKENE HYDROCARBONS WITH A DOUBLE C=C BOND ARE CALLED ALKENES

CRACKING WHAT HAPPENS? MANY DIFFERENT HYDROCARBONS ARE PRODUCED BECAUSE THE MOLECULES CAN BREAK DOWN IN DIFFERENT WAYS ALKANE ALKENE HYDROCARBONS WITH SINGLE C-C BONDS ARE CALLED ALKANES ALKANE ALKENE HYDROCARBONS WITH A DOUBLE C=C BOND ARE CALLED ALKENES

CRUDEOIL THE END © 2011 JONATHAN HOPTON & KNOCKHARDY PUBLISHING