CRT 2010 Washington DC January 21 2010 The








- Slides: 19

CRT 2010 Washington DC, January 21, 2010 The durability of Transcatheter Valves should be the same as Surgically Implanted Valves? Eberhard Grube, MD, FACC, FSCAI St. Elisabeth Hospital, Essen, Germany Heart Center Rhein-Ruhr Instituto Cardiologico Dante Pazzanese, São Paulo, Brazil

DISCLOSURES Eberhard Grube, MD Consulting Fees – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson Company, Medtronic Cardio. Vascular, Inc. Honoraria – Biosensors International , Boston Scientific Corporation, Medtronic Cardio. Vascular, Inc Ownership Interest (Stocks, Stock Options or Other Ownership Interest) – Biosensors International , Medtronic Cardio. Vascular, Inc. I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference off-label use of stents and valve prosthesis.

Surgical Replacement is the Gold Standard today ● But the “Gold Standard’ has seen evolution over the years: 1 - First Mechanical Valves 2 - Then Biological Tissue Valves 3 - And in the Future, Transcatheter Valves? ● We need to leverage learning from these last two waves of evolution in order to not repeat the mistakes of the past

Surgical Replacement Durability in Context ● Valves released with equivalent of 5 years on accelerated wear tester (200 M cycles) ● Current valves on the market are 3 rd, 4 th, or 5 th generation from the various manufacturers ● Pericardial tissue valves have 20 years of patient experience

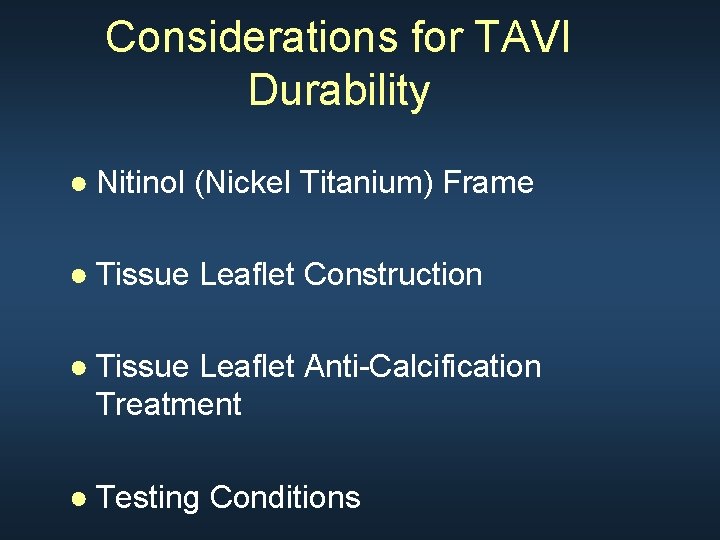
Considerations for TAVI Durability ● Nitinol (Nickel Titanium) Frame ● Tissue Leaflet Construction ● Tissue Leaflet Anti-Calcification Treatment ● Testing Conditions

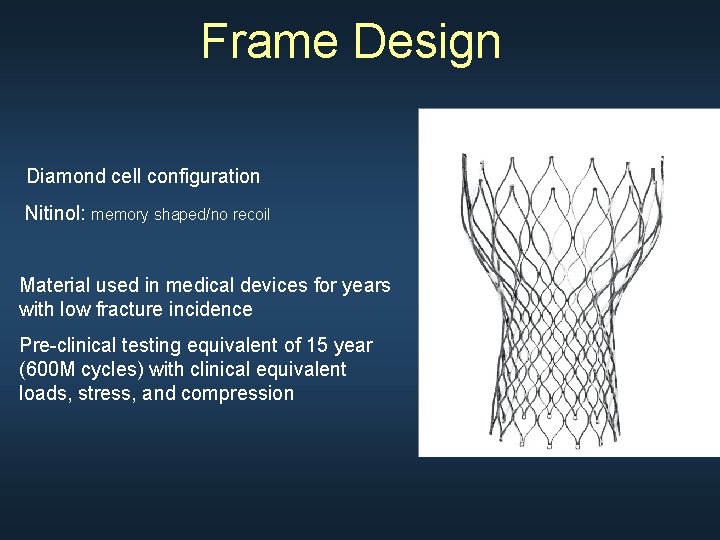
Frame Design Diamond cell configuration Nitinol: memory shaped/no recoil Material used in medical devices for years with low fracture incidence Pre-clinical testing equivalent of 15 year (600 M cycles) with clinical equivalent loads, stress, and compression

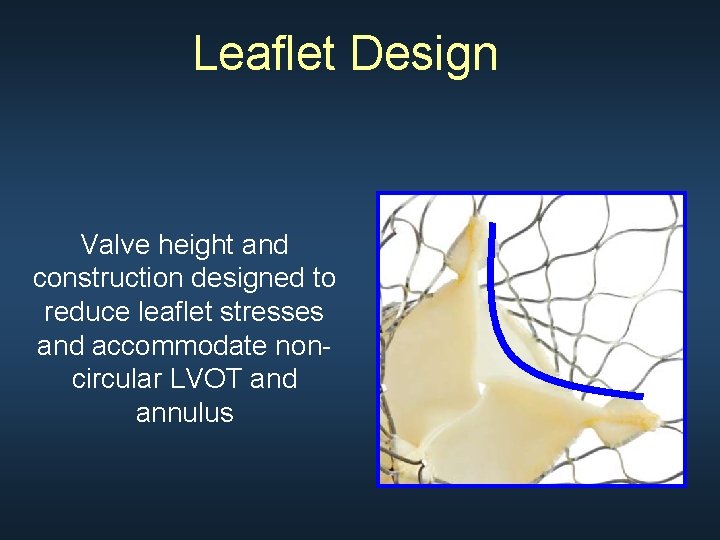
Leaflet Design Valve height and construction designed to reduce leaflet stresses and accommodate noncircular LVOT and annulus

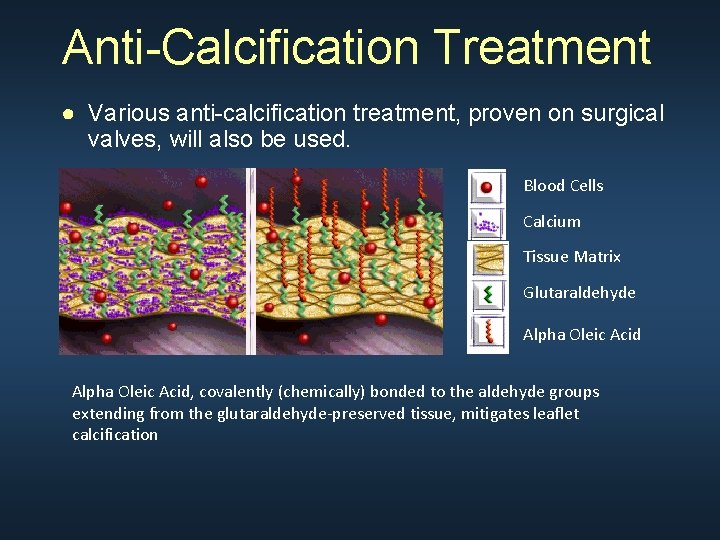
Anti-Calcification Treatment ● Various anti-calcification treatment, proven on surgical valves, will also be used. Blood Cells Calcium Tissue Matrix Glutaraldehyde Alpha Oleic Acid, covalently (chemically) bonded to the aldehyde groups extending from the glutaraldehyde-preserved tissue, mitigates leaflet calcification

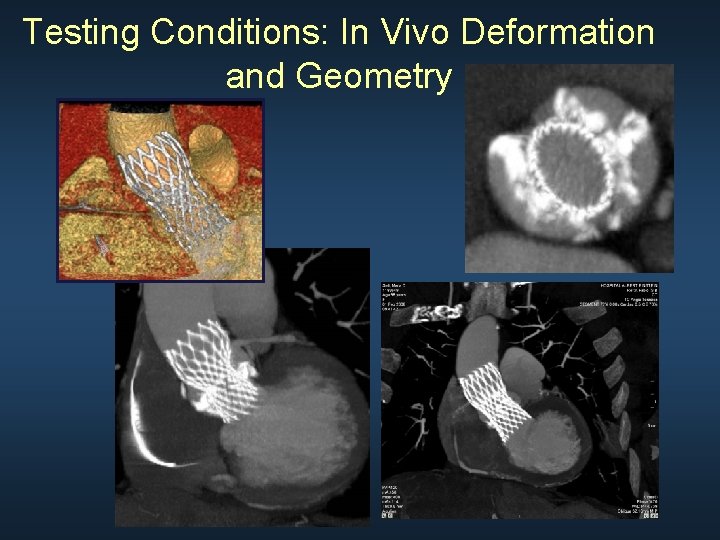
Testing Conditions: In Vivo Deformation and Geometry

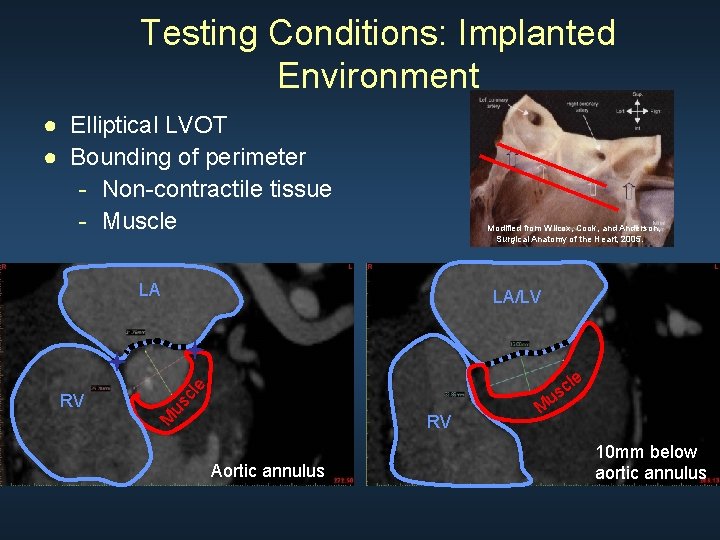
Testing Conditions: Implanted Environment ● Elliptical LVOT ● Bounding of perimeter - Non-contractile tissue - Muscle Modified from Wilcox, Cook, and Anderson, Surgical Anatomy of the Heart, 2005. LA us cle le RV M RV LA/LV Aortic annulus M c us 10 mm below aortic annulus

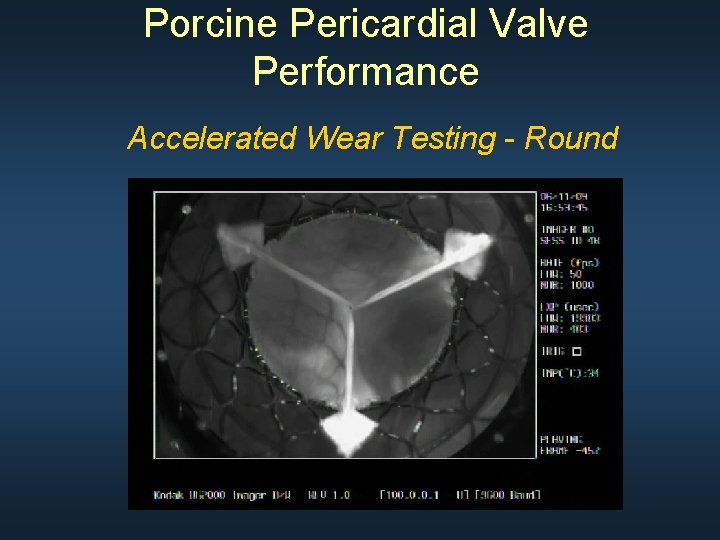
Porcine Pericardial Valve Performance Accelerated Wear Testing (200 millions cycles: 5 years)

Porcine Pericardial Valve Performance Accelerated Wear Testing - Round

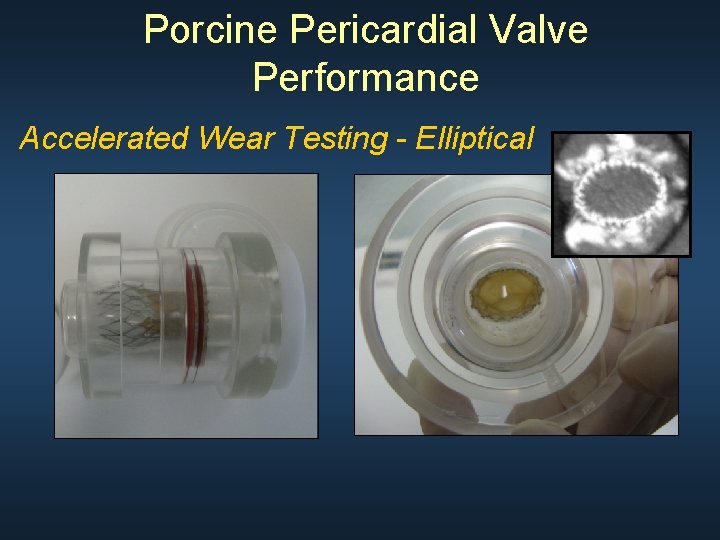
Porcine Pericardial Valve Performance Accelerated Wear Testing - Elliptical

Porcine Pericardial Valve Performance Accelerated Wear Testing - Elliptical

Durability is a relative concept ● Life Expectancy with Therapy (will the device outlive the patient in any case? ) ● Has to be taken into account with the following considerations: - Patient’s co-morbidities Procedure invasiveness Post-operative Length of Stay Safety Efficacy Quality of Life Cost Effectiveness ● Valve in Valve concept opens new opportunity to: - Extend durability without risk and invasiveness of open surgery - Bank on future innovations and iterations

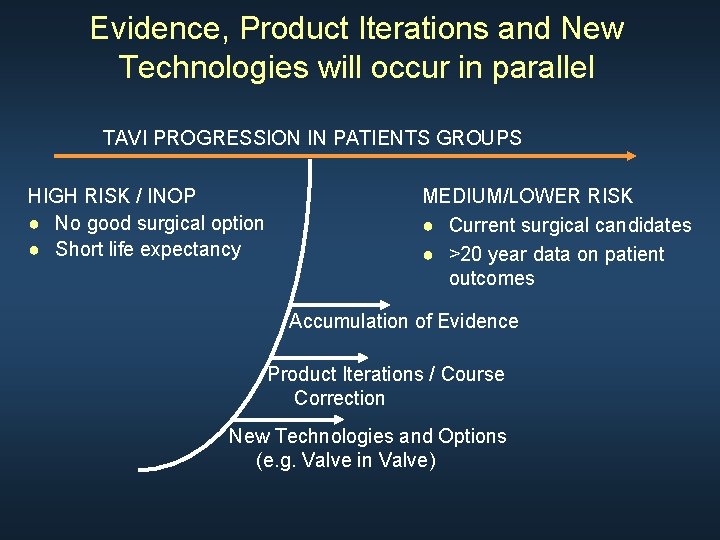
As TAVI experience grows the landscape will shift over the years TAVI PROGRESSION IN PATIENTS GROUPS HIGH RISK / INOP ● No good surgical option ● Short life expectancy MEDIUM/LOWER RISK ● Current surgical candidates ● >20 year data on patient outcomes

Evidence, Product Iterations and New Technologies will occur in parallel TAVI PROGRESSION IN PATIENTS GROUPS HIGH RISK / INOP ● No good surgical option ● Short life expectancy MEDIUM/LOWER RISK ● Current surgical candidates ● >20 year data on patient outcomes Accumulation of Evidence Product Iterations / Course Correction New Technologies and Options (e. g. Valve in Valve)

So what do we know about Durability of TAVI today? ● Bench testing can provide key insight into the durability of the design - Equivalency with surgical valves established through accelerated wear testing, an FDA requirement - Frame accelerated wear testing completed up to 15 years ● With over 7, 500 patients implanted to date, - No reports of premature ageing of the leaflets - No frame fracture of the nitinol frame ● Survival experience continues to accumulate, with the longest surviving patient being implanted in 2004

So in brief: ● YES, TAVI should strive to match Surgical on Durability, especially if we want it to displace Surgical from the Medium/Lower risk patient group ● NO, it doesn’t need to happen all at once, it will happen over time, through evidence gathering, product iterations and new technologies
 Crt 2010
Crt 2010 Direct view storage tube in computer graphics
Direct view storage tube in computer graphics Monitor crt lcd led
Monitor crt lcd led Fosdick test
Fosdick test Focusing system in crt
Focusing system in crt Crt
Crt Digital cockpit display unit
Digital cockpit display unit Diagram of cathode ray tube
Diagram of cathode ray tube Partes de un monitor crt
Partes de un monitor crt Nrt and crt examples
Nrt and crt examples Pengertian monitor crt
Pengertian monitor crt Crt 2017
Crt 2017 Uses crt; var
Uses crt; var Uses crt
Uses crt Types of crt
Types of crt Crt lcd led
Crt lcd led Crt aptitude
Crt aptitude Crt graphics
Crt graphics Budowa monitora
Budowa monitora Crt monitor
Crt monitor