CRT 2010 Washington DC January 21 2010 Selfexpanding






- Slides: 19

CRT 2010 Washington DC, January 21, 2010 Self-expanding Subclavian Approach: Techniques and Outcomes Eberhard Grube, MD, FACC, FSCAI St. Elisabeth Hospital, Heart Center Rhein-Ruhr, Essen, Germany Instituto Cardiologico Dante Pazzanese, São Paulo, Brazil

DISCLOSURES Eberhard Grube, MD Consulting Fees – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson Company, Medtronic Cardio. Vascular, Inc. Honoraria – Biosensors International , Boston Scientific Corporation, Medtronic Cardio. Vascular, Inc Ownership Interest (Stocks, Stock Options or Other Ownership Interest) – Biosensors International , Medtronic Cardio. Vascular, Inc. I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference off-label use of stents and valve prosthesis.

Subclavian as an Alternative Access ● Provides an alternate access for both cardiac surgeons and interventional cardiologists ● Enables implanting teams to mitigate risk by better adapting procedure to patient’s anatomy ● Training for subclavian is incremental for both transapical and transfemoral implanters

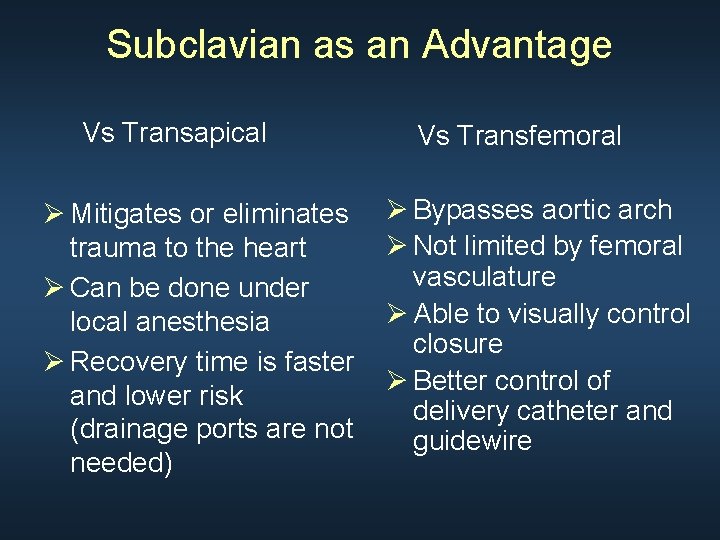
Subclavian as an Advantage Vs Transapical Ø Mitigates or eliminates trauma to the heart Ø Can be done under local anesthesia Ø Recovery time is faster and lower risk (drainage ports are not needed) Vs Transfemoral Ø Bypasses aortic arch Ø Not limited by femoral vasculature Ø Able to visually control closure Ø Better control of delivery catheter and guidewire

Procedure Access Overall Experience Carotid Axillary/Subclavian < 0. 1 % _ 7% < 0. 1 % Trans aorta < 0. 1_ % Transapical Medtronic Core. Valve Edwards-Sapien Transfemoral approach > 90 % < 10 % <1% > 30 % < 0. 1 % 60 %

Subclavian Access is Growing Globally (Expanded Evaluation Registry) * EER reporting is voluntary and is not indicative of total cases

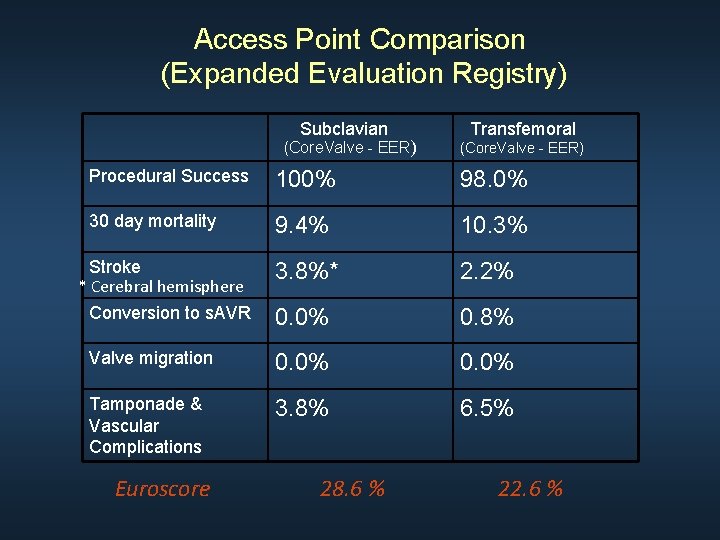
Access Point Comparison (Expanded Evaluation Registry) Subclavian (Core. Valve - EER) Transfemoral (Core. Valve - EER) Procedural Success 100% 98. 0% 30 day mortality 9. 4% 10. 3% 3. 8%* 2. 2% Conversion to s. AVR 0. 0% 0. 8% Valve migration 0. 0% Tamponade & Vascular Complications 3. 8% 6. 5% Stroke * Cerebral hemisphere Euroscore 28. 6 % 22. 6 %

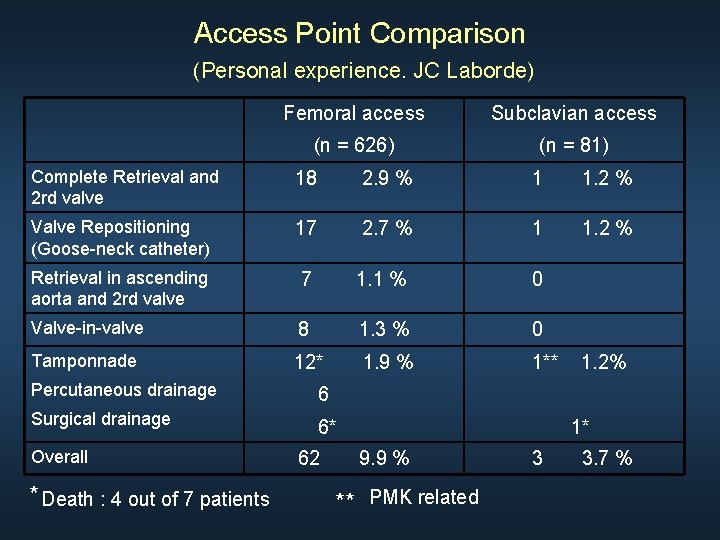
Access Point Comparison (Personal experience. JC Laborde) Femoral access Subclavian access (n = 626) (n = 81) Complete Retrieval and 2 rd valve 18 2. 9 % 1 1. 2 % Valve Repositioning (Goose-neck catheter) 17 2. 7 % 1 1. 2 % Retrieval in ascending aorta and 2 rd valve 7 1. 1 % 0 Valve-in-valve 8 1. 3 % 0 Tamponnade 12* 1. 9 % 1** Percutaneous drainage 6 Surgical drainage 6* Overall * Death : 4 out of 7 patients 62 1. 2% 1* 9. 9 % ** PMK related 3 3. 7 %

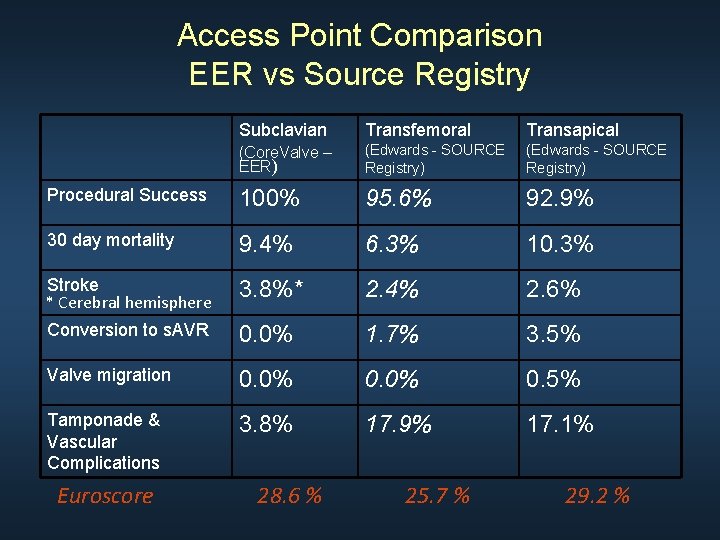
Access Point Comparison EER vs Source Registry Subclavian Transfemoral Transapical (Core. Valve – EER) (Edwards - SOURCE Registry) Procedural Success 100% 95. 6% 92. 9% 30 day mortality 9. 4% 6. 3% 10. 3% Stroke * Cerebral hemisphere 3. 8%* 2. 4% 2. 6% Conversion to s. AVR 0. 0% 1. 7% 3. 5% Valve migration 0. 0% 0. 5% Tamponade & Vascular Complications 3. 8% 17. 9% 17. 1% Euroscore 28. 6 % 25. 7 % 29. 2 %

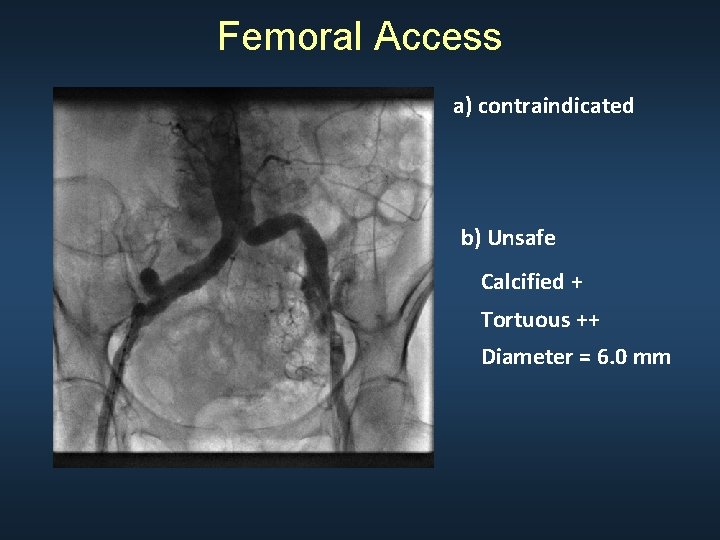
Femoral Access a) contraindicated b) Unsafe Calcified + Tortuous ++ Diameter = 6. 0 mm

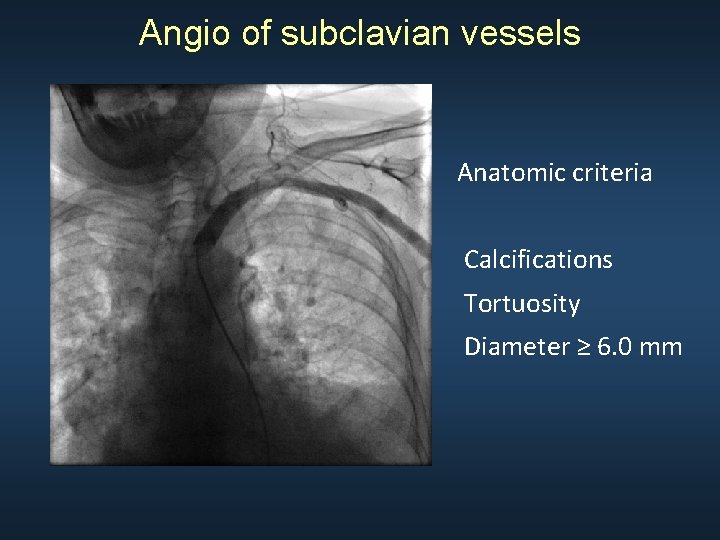
Angio of subclavian vessels Anatomic criteria Calcifications Tortuosity Diameter ≥ 6. 0 mm

Angio of Aortic route Feasibility & Safety Calcifications + Tortuosity ++

Core. Valve TAVR subclavian/trans-axillary Set-Up • General anesthesia • Transoesophagial Echocardiography (T. E. E. ) • Cath-Lab • Clopidrogrel/Aspirin (including loading dose) • Antibiotic prophylaxis

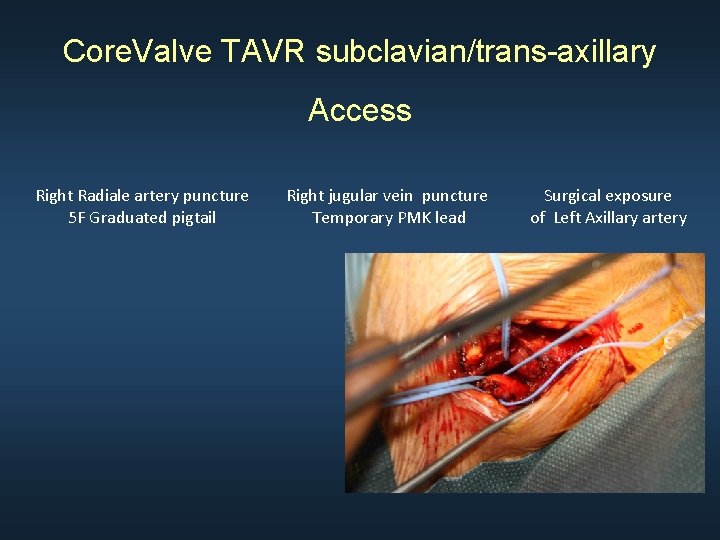
Core. Valve TAVR subclavian/trans-axillary Access Right Radiale artery puncture 5 F Graduated pigtail Right jugular vein puncture Temporary PMK lead Surgical exposure of Left Axillary artery

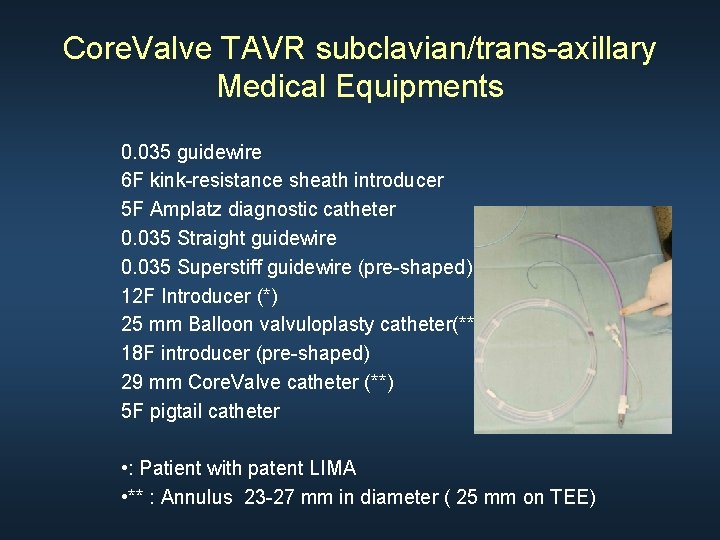
Core. Valve TAVR subclavian/trans-axillary Medical Equipments 0. 035 guidewire 6 F kink-resistance sheath introducer 5 F Amplatz diagnostic catheter 0. 035 Straight guidewire 0. 035 Superstiff guidewire (pre-shaped) 12 F Introducer (*) 25 mm Balloon valvuloplasty catheter(**) 18 F introducer (pre-shaped) 29 mm Core. Valve catheter (**) 5 F pigtail catheter • : Patient with patent LIMA • ** : Annulus 23 -27 mm in diameter ( 25 mm on TEE)

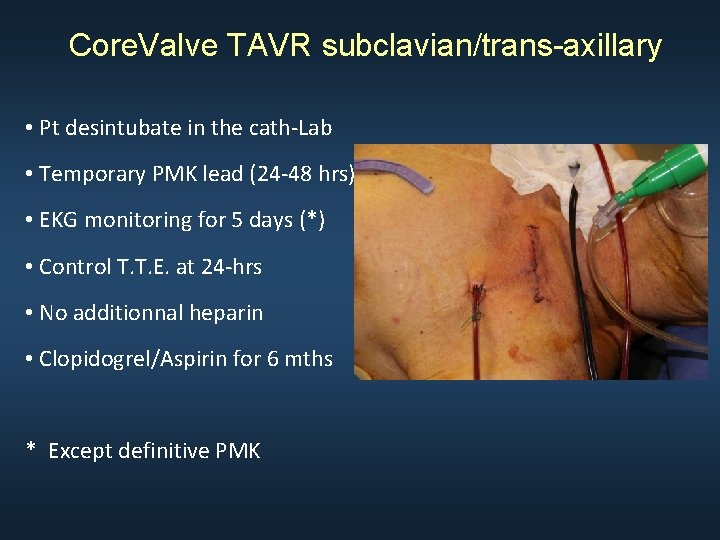
Core. Valve TAVR subclavian/trans-axillary • Pt desintubate in the cath-Lab • Temporary PMK lead (24 -48 hrs) • EKG monitoring for 5 days (*) • Control T. T. E. at 24 -hrs • No additionnal heparin • Clopidogrel/Aspirin for 6 mths * Except definitive PMK

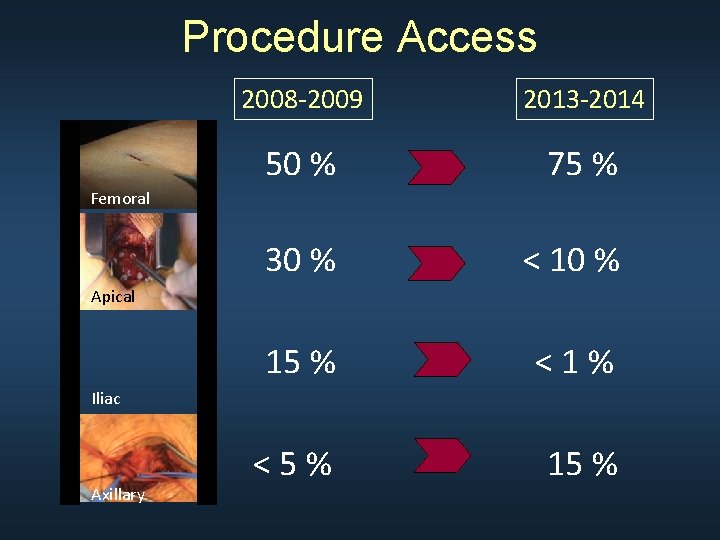
Procedure Access 2008 -2009 2013 -2014 50 % 75 % 30 % < 10 % 15 % <1% <5% 15 % Femoral Apical Iliac Axillary

Conclusion Subclavian is a lower risk alternative to both transapical and transfemoral for many patients and should be considered in order to improve patient outcomes.

Thank you for your attention !
 Crt 2010
Crt 2010 Převod vhs do pc
Převod vhs do pc Uses crt;
Uses crt; Crt micron manuale italiano
Crt micron manuale italiano Difference between crt and dvst
Difference between crt and dvst Var a b integer
Var a b integer Crt l
Crt l Owl purdue literary criticism
Owl purdue literary criticism Crts full form in medical ryles tube
Crts full form in medical ryles tube Crt
Crt Crt display technology
Crt display technology Rsa crt calculator
Rsa crt calculator Mantenimiento monitor crt
Mantenimiento monitor crt Lvcm medtronic
Lvcm medtronic Crt roanne
Crt roanne Nrt and crt examples
Nrt and crt examples Race theory definition
Race theory definition Refresh crt consist of
Refresh crt consist of Monitory crt
Monitory crt Crt buffer test
Crt buffer test