Crossreactivity of Angioedema Between ACEIs and ARBs Jimmy

Cross-reactivity of Angioedema Between ACEIs and ARBs Jimmy Gonzalez, Pharm. D. PGY-2 Drug Information Resident Robert Wood Johnson University Hospital May 2016

Financial Disclosures Neither the presenter nor the planning committee has received any commercial support in the development of this educational activity. 2

Learning Objectives • Explain proposed mechanisms for cross-reactivity between ACEIs and ARBs • Describe the risk of angioedema with ARBs following ACEI-induced angioedema 3

Case Vignette BB is a 56 -year old white male with a prior medical history significant for hypertension, chronic kidney disease, diabetes, and coronary artery disease. He currently takes atorvastatin, metformin, sitagliptin, sevelamer, and telmisartan. He experienced multiple minor episodes of lip and tongue swelling while taking ramipril in the past. How likely is he to develop angioedema secondary to any of his current medications? 4
What is Angioedema? • Localized, nonpitting and nonpruritic swelling • Extravasation of plasma into subcutaneous tissue • Common locations: – – Face Lips Tongue Respiratory tract Rarely: intestinal tract • Induced by increased formation or reduced clearance of vasoactive peptides Hoover T. Clin Exp Allergy. 2010; 40(1): 50 -61. 5
Risk Factors for Angioedema • • • African American race Heart failure Female gender Smoking Increased age Hoover T. Clin Exp Allergy. 2010; 40(1): 50 -61. 6
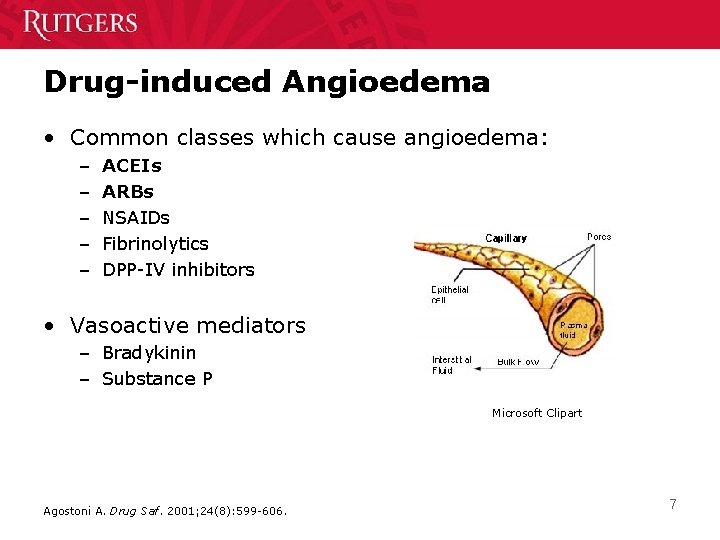
Drug-induced Angioedema • Common classes which cause angioedema: – – – ACEIs ARBs NSAIDs Fibrinolytics DPP-IV inhibitors • Vasoactive mediators – Bradykinin – Substance P Microsoft Clipart Agostoni A. Drug Saf. 2001; 24(8): 599 -606. 7
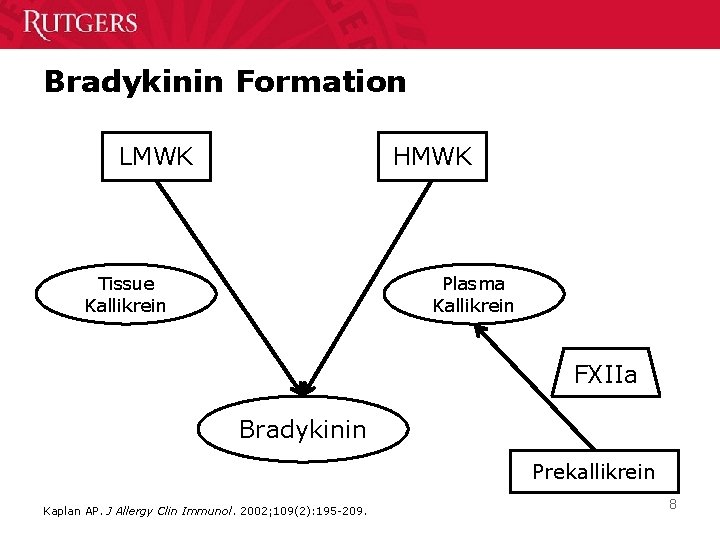
Bradykinin Formation LMWK HMWK Tissue Kallikrein Plasma Kallikrein FXIIa Bradykinin Prekallikrein Kaplan AP. J Allergy Clin Immunol. 2002; 109(2): 195 -209. 8
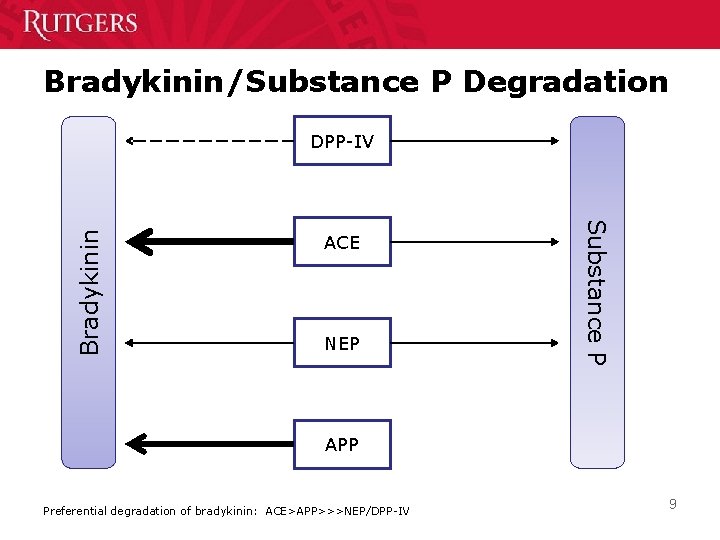
Bradykinin/Substance P Degradation ACE NEP Substance P Bradykinin DPP-IV APP Preferential degradation of bradykinin: ACE>APP>>>NEP/DPP-IV 9
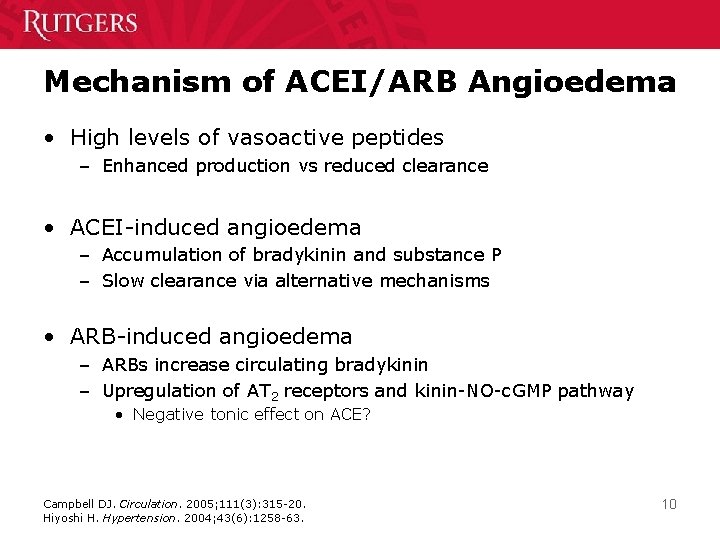
Mechanism of ACEI/ARB Angioedema • High levels of vasoactive peptides – Enhanced production vs reduced clearance • ACEI-induced angioedema – Accumulation of bradykinin and substance P – Slow clearance via alternative mechanisms • ARB-induced angioedema – ARBs increase circulating bradykinin – Upregulation of AT 2 receptors and kinin-NO-c. GMP pathway • Negative tonic effect on ACE? Campbell DJ. Circulation. 2005; 111(3): 315 -20. Hiyoshi H. Hypertension. 2004; 43(6): 1258 -63. 10

ACEI-induced Angioedema ACE NEP Substance P Bradykinin DPP-IV APP Duan QL. Am J Hum Genet. 2005; 77(4): 617 -26. 11
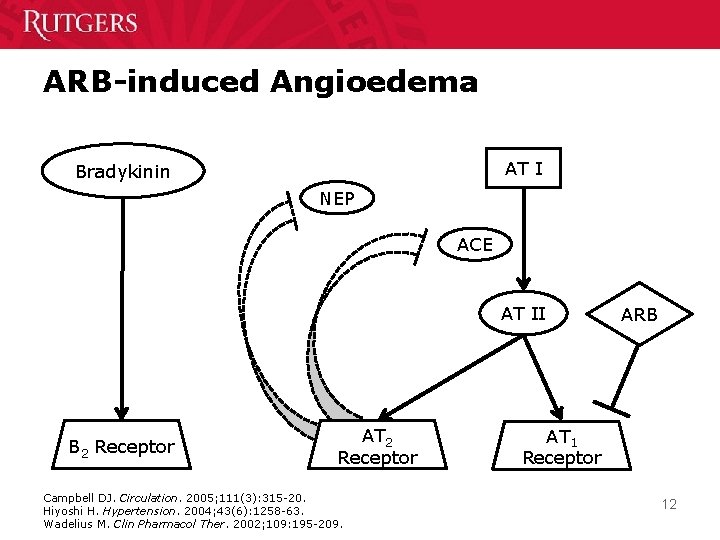
ARB-induced Angioedema AT I Bradykinin NEP ACE AT II B 2 Receptor AT 2 Receptor Campbell DJ. Circulation. 2005; 111(3): 315 -20. Hiyoshi H. Hypertension. 2004; 43(6): 1258 -63. Wadelius M. Clin Pharmacol Ther. 2002; 109: 195 -209. ARB AT 1 Receptor 12
![Native Incidence of Angioedema ACEI-induced 0. 3% [0. 28 -0. 32%] ARB-induced 0. 11% Native Incidence of Angioedema ACEI-induced 0. 3% [0. 28 -0. 32%] ARB-induced 0. 11%](http://slidetodoc.com/presentation_image_h2/775abcbce96fe4fcc8345d31077ead61/image-13.jpg)
Native Incidence of Angioedema ACEI-induced 0. 3% [0. 28 -0. 32%] ARB-induced 0. 11% [0. 09 -0. 13%] Makani H. Am J Cardiol. 2012; 110(3): 383 -91. 13
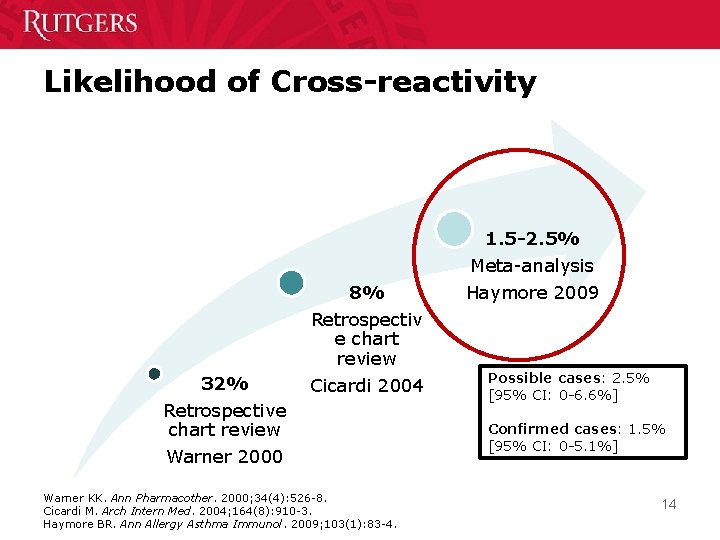
Likelihood of Cross-reactivity 1. 5 -2. 5% 8% 32% Retrospectiv e chart review Cicardi 2004 Retrospective chart review Warner 2000 Warner KK. Ann Pharmacother. 2000; 34(4): 526 -8. Cicardi M. Arch Intern Med. 2004; 164(8): 910 -3. Haymore BR. Ann Allergy Asthma Immunol. 2009; 103(1): 83 -4. Meta-analysis Haymore 2009 Possible cases: 2. 5% [95% CI: 0 -6. 6%] Confirmed cases: 1. 5% [95% CI: 0 -5. 1%] 14

DPP-IV: INTERACTIONS AS WELL? 15
DPP-IV Functions • Cell-surface endopeptidase – Found throughout the body • Numerous substrates – Incretins (e. g. , GLP-1) – Substance P – Des-Arg 9–bradykinin • Importance may grow with ACE, angiotensin receptor, or neprilysin co-inhibition Mentlein R. Regul Pept. 1999; 85(1): 9 -24. 16
DPP-IV Angioedema • Decreased DPP-IV activity noted during acute angioedema attacks – Compared to normotensive, remote angioedema, untreated hypertensive patients • Low contribution on bradykinin metabolism – Angioedema likely Substance P-mediated • Risk enhanced by: – Concomitant ACEIs – Acquired or genetic DPP-IV deficiency Byrd JB. Hypertension. 2008; 51: 141– 7. Lefebvre J. Hypertension. 2002; 39[part 2]: 460 -4. 17
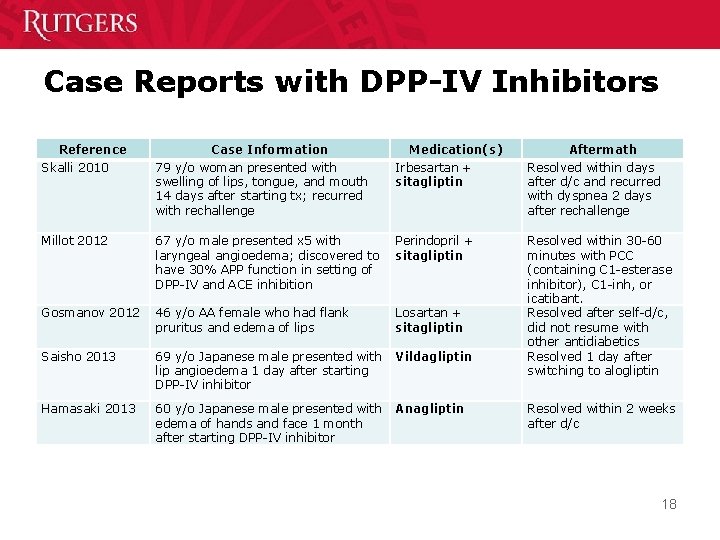
Case Reports with DPP-IV Inhibitors Reference Skalli 2010 Case Information 79 y/o woman presented with swelling of lips, tongue, and mouth 14 days after starting tx; recurred with rechallenge Medication(s) Irbesartan + sitagliptin Aftermath Resolved within days after d/c and recurred with dyspnea 2 days after rechallenge Millot 2012 67 y/o male presented x 5 with laryngeal angioedema; discovered to have 30% APP function in setting of DPP-IV and ACE inhibition Perindopril + sitagliptin Gosmanov 2012 46 y/o AA female who had flank pruritus and edema of lips Losartan + sitagliptin Saisho 2013 69 y/o Japanese male presented with lip angioedema 1 day after starting DPP-IV inhibitor Vildagliptin Resolved within 30 -60 minutes with PCC (containing C 1 -esterase inhibitor), C 1 -inh, or icatibant. Resolved after self-d/c, did not resume with other antidiabetics Resolved 1 day after switching to alogliptin Hamasaki 2013 60 y/o Japanese male presented with edema of hands and face 1 month after starting DPP-IV inhibitor Anagliptin Resolved within 2 weeks after d/c 18
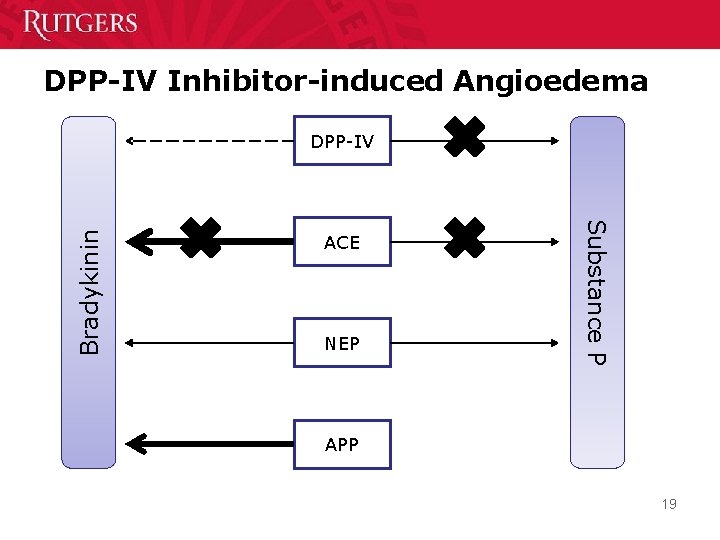
DPP-IV Inhibitor-induced Angioedema ACE NEP Substance P Bradykinin DPP-IV APP 19
Conclusions • The relative incidence of angioedema with – ACEIs: 0. 3% – ARBs: 0. 11% – Both: 1. 5 -2. 5% (cross-reactivity) • Likelihood of cross-reactivity: <10% chance • Angioedema risk with DPP-IV inhibitors may be higher with ACEI/ARBs 20

Case Vignette BB is a 56 -year old white male with a prior medical history significant for hypertension, chronic kidney disease, diabetes, and coronary artery disease. He currently takes atorvastatin, metformin, sitagliptin, sevelamer, and telmisartan. He experienced multiple minor episodes of lip and tongue swelling while taking ramipril in the past. 21
Assessment Question 1 • What is the likelihood of BB developing angioedema from telmisartan given a history of angioedema with ramipril? A. B. C. D. E. 0% <10% 20 -30% 40 -60% >60% 22
Assessment Question 1 • What is the likelihood of BB developing angioedema from telmisartan given a history of angioedema with ramipril? A. B. C. D. E. 0% <10% 20 -30% 40 -60% >60% 23
Assessment Question 2 • Which of the following statements is correct regarding ACE/ARB/DPP-IV induced angioedema? A. Bradykinin levels are elevated in both ACEI and ARBangioedema B. Substance P levels may be elevated with DPP-IV inhibitors C. ARBs may indirectly inhibit ACE and NEP activity D. DPP-IV primarily inactivates Substance P E. All of the above 24
Assessment Question 2 • Which of the following statements is correct regarding ACE/ARB/DPP-IV induced angioedema? A. Bradykinin levels are elevated in both ACEI and ARBangioedema B. Substance P levels are elevated with DPP-IV inhibitor use C. ARBs may indirectly inhibit ACE and NEP activity D. DPP-IV primarily inactivates Substance P E. All of the above 25

Questions? Thank you! 26

References • • • • • Hoover T, Lippmann M, Grouzmann E, et al. Angiotensin converting enzyme inhibitor induced angio-oedema: A review of the pathophysiology and risk factors. Clin Exp Allergy. 2010; 40(1): 50 -61. Agostoni A, Cicardi M. Drug-induced angioedema without urticaria. Drug Saf. 2001; 24(8): 599 -606. Kaplan AP, Joseph K, Silverberg M. Pathways for bradykinin formation and inflammatory disease. J Allergy Clin Immunol. 2002; 109(2): 195 -209. Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation. 2005; 111(3): 315 -20. Hiyoshi H, Yayama K, Takano M, Okamoto H. Stimulation of cyclic GMP production via AT 2 and B 2 receptors in the pressure-overloaded aorta after banding. Hypertension. 2004; 43(6): 1258 -63. Duan QL, Nikpoor B, Dube MP, et al. A variant in XPNPEP 2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005; 77(4): 617 -26. Wadelius M, Marshall SE, Islander G, et al. Phenotype standardization of angioedema in the head and neck region caused by agents acting on the angiotensin system. Clin Pharmacol Ther. 2014; 96(4): 477 -81. Makani H, Messerli FH, Romero J, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin-angiotensin system inhibitors. Am J Cardiol. 2012; 110(3): 383 -91. Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother. 2000; 34(4): 526 -8. Haymore BR, De. Zee KJ. Use of angiotensin receptor blockers after angioedema with an angiotensin-converting enzyme inhibitor. Ann Allergy Asthma Immunol. 2009; 103(1): 83 -4. Cicardi M, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004; 164(8): 910 -3. Mentlein R. Dipeptidyl-peptidase IV (CD 26)--role in the inactivation of regulatory peptides. Regul Pept. 1999; 85(1): 9 -24. Byrd JB, Touzin K, Sile S, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008; 51(1): 141 -7. Lefebvre J, Murphey LJ, Hartert TV, et al. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension. 2002; 39[part 2]: 460 -4. Skalli S, Wion-barbot N, Baudrant M, et al. Angio-oedema induced by dual dipeptidyl peptidase inhibitor and angiotensin II receptor blocker: A first case report. Diabet Med. 2010; 27(4): 486 -7. Millot I, Plancade D, Hosotte M, et al. Treatment of a life-threatening laryngeal bradykinin angio-oedema precipitated by dipeptidylpeptidase-4 inhibitor and angiotensin-I converting enzyme inhibitor with prothrombin complex concentrates. Br J Anaesth. 2012; 109(5): 827 -9. Gosmanov AR, Fontenot EC. Sitagliptin-associated angioedema. Diabetes Care. 2012; 35(8): e 60. Saisho Y, Itoh H. Dipeptidyl peptidase-4 inhibitors and angioedema: a class effect? . Diabet Med. 2013; 30(4): e 149 -50. Hamasaki H, Yanai H. The development of angioedema in a patient with type 2 diabetes due to a novel dipeptidyl peptidase-IV inhibitor, anagliptin. Int J Cardiol. 2013; 168(3): e 106. 27
- Slides: 27