Critical Appraisal of the ISCHEMIA Trial Gregg W


- Slides: 31

Critical Appraisal of the ISCHEMIA Trial Gregg W. Stone, MD The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, NY and the Cardiovascular Research Foundation Scientific. Sessions. org#AHA 19

General Disclosure Statement of Financial Interest Speaker honoraria from Cook; Consultant to Valfix, Ther. Ox, Robocath, Heart. Flow, Ablative Solutions, Miracor, Neovasc, Abiomed, Ancora, Vectorious; Equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, Spectra. Wave, Orchestra Biomed, Aria, Cardiac Success, Valfix Relevant Financial Disclosures Supported by NHLBI grants Consultant to Heart. Flow Scientific. Sessions. org#AHA 19

ISCHEMIA Trial: Background • Prior trials (most notably COURAGE and BARI-2 D) did not show that revascularization in stable CAD prevents death or MI • Limitations: - Enrolled after angiography → Low risk pts enrolled - Highest risk anatomical pts were excluded - Most pts had minimal to only moderate ischemia • Did not use contemporary stents, physiologic guidance, bilateral IMA, pharmacotherapy, etc. Scientific. Sessions. org#AHA 19

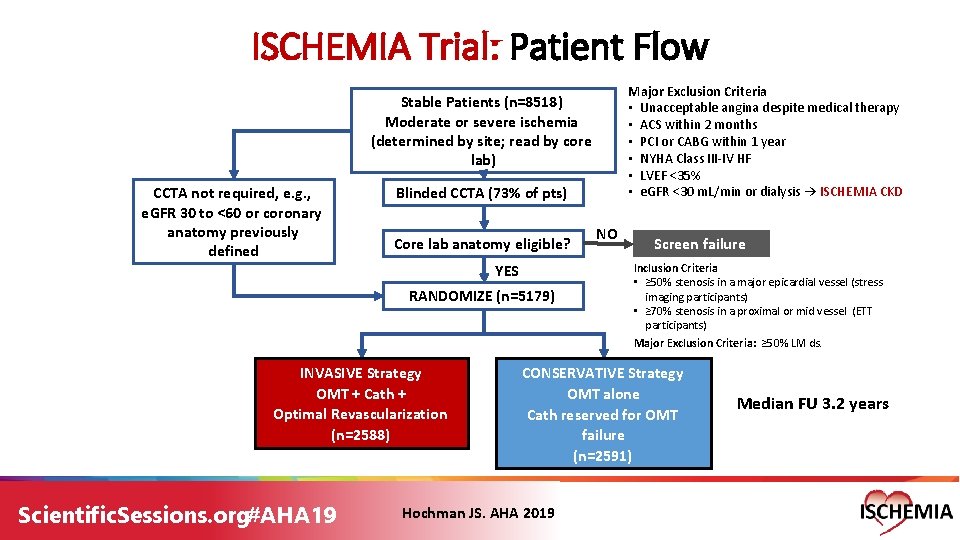
ISCHEMIA Trial: Patient Flow Major Exclusion Criteria • Unacceptable angina despite medical therapy • ACS within 2 months • PCI or CABG within 1 year • NYHA Class III-IV HF • LVEF <35% • e. GFR <30 m. L/min or dialysis → ISCHEMIA CKD Stable Patients (n=8518) Moderate or severe ischemia (determined by site; read by core lab) CCTA not required, e. g. , e. GFR 30 to <60 or coronary anatomy previously defined Blinded CCTA (73% of pts) Core lab anatomy eligible? YES RANDOMIZE (n=5179) INVASIVE Strategy OMT + Cath + Optimal Revascularization (n=2588) Scientific. Sessions. org#AHA 19 NO Screen failure Inclusion Criteria • ≥ 50% stenosis in a major epicardial vessel (stress imaging participants) • ≥ 70% stenosis in a proximal or mid vessel (ETT participants) Major Exclusion Criteria: ≥ 50% LM ds. CONSERVATIVE Strategy OMT alone Cath reserved for OMT failure (n=2591) Hochman JS. AHA 2019 Median FU 3. 2 years

ISCHEMIA Leadership National Heart Lung & Blood Institute: Yves Rosenberg, Jerome Fleg, Neal Jeffries, Ruth Kirby Clinical Coordinating Center: NYU Cardiovascular Clinical Research Center Harmony Reynolds Sripal Bangalore Jeffrey Berger, Jonathan Newman Stephanie Mavromichalis Mandeep Sidhu (Albany Medical Ctr) Imaging Coordinating Center : Leslee Shaw (Emory/Weil Cornell Medicine) Top Countries/Regions Leaders: Balram Bhargava (India), Roxy Senior (UK), Shaun Goodman, Gilbert Gosselin (Canada), Renato Lopes (Brazil), Witold Ruzyllo, Hanna Szwed (Poland), Leo Bockeria (Russia), José Lopez-Sendon (Spain), Aldo Maggioni (Italy), Harvey White (Singapore, New Zealand), Rolf Doerr (Germany) Scientific. Sessions. org#AHA 19 Study Chair: Judith S. Hochman (New York University) Study Co-Chair: David J. Maron (Stanford University) Executive Committee: Leadership Committee: Judith Hochman, Chair David Maron, Co-Chair William Boden Bruce Ferguson Robert Harrington Gregg W. Stone* David Williams Karen Alexander Sripal Bangalore Jeffrey Berger Daniel Mark Sean O’Brien Harmony Reynolds Yves Rosenberg Leslee Shaw John Spertus Statistical and Data Coordinating Center: Duke Clinical Research Institute Sean O’Brien Karen Alexander Lisa Hatch Frank Harrell (Vanderbilt) EQOL Coordinating Center: Daniel Mark (Duke University) John Spertus (St. Luke’s Mid America Heart Institute) Data Safety Monitoring Board: Lawrence Friedman, Chair; Jeffrey Anderson; Jessica Berg; David De. Mets; C. Michael Gibson; Gervasio A. Lamas; Pamela Ouyang; Pamela K. Woodard Clinical Event Adjudication Committee Chair: Bernard Chaitman (Saint Louis University) *Chair of the PCI and CABG revascularization committee

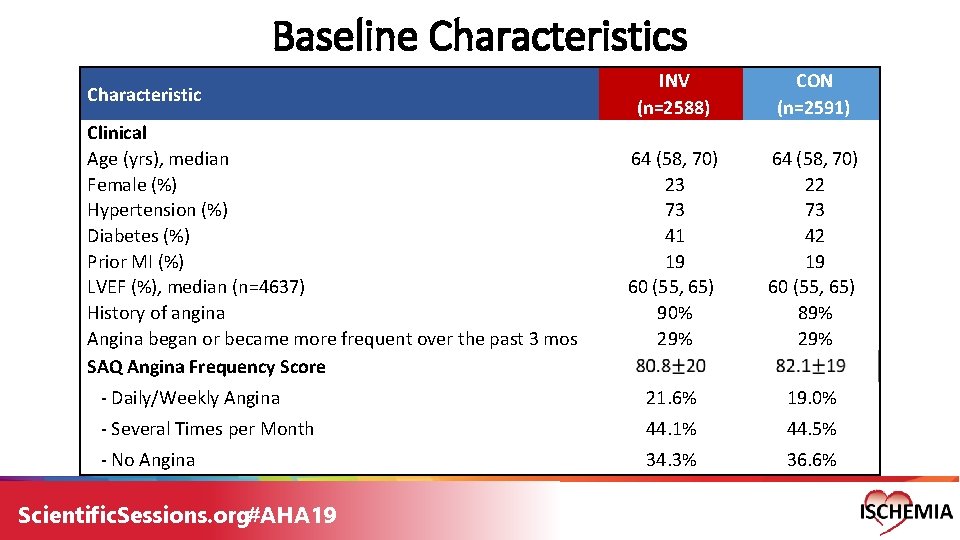
Baseline Characteristics INV (n=2588) CON (n=2591) 64 (58, 70) 23 73 41 19 60 (55, 65) 90% 29% 64 (58, 70) 22 73 42 19 60 (55, 65) 89% 29% - Daily/Weekly Angina 21. 6% 19. 0% - Several Times per Month 44. 1% 44. 5% - No Angina 34. 3% 36. 6% Characteristic Clinical Age (yrs), median Female (%) Hypertension (%) Diabetes (%) Prior MI (%) LVEF (%), median (n=4637) History of angina Angina began or became more frequent over the past 3 mos SAQ Angina Frequency Score Scientific. Sessions. org#AHA 19

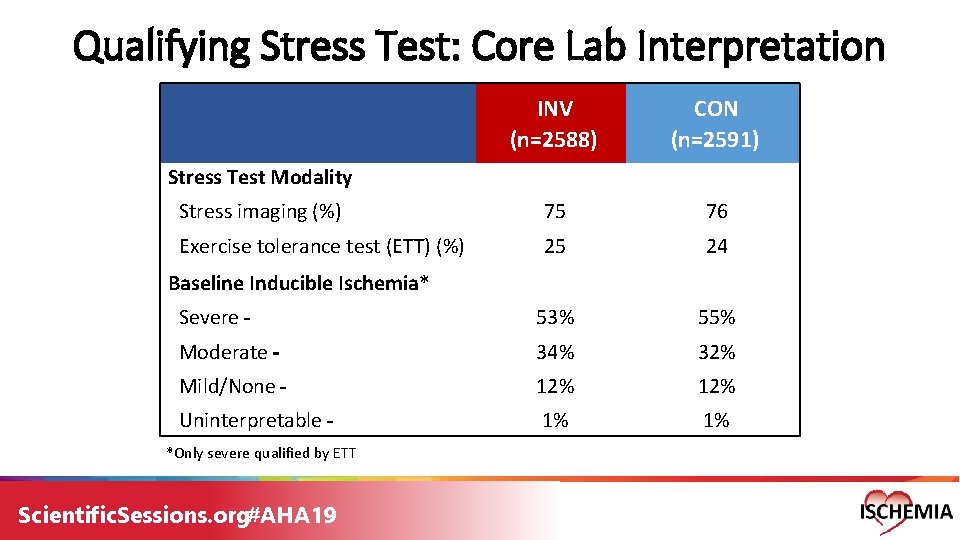
Qualifying Stress Test: Core Lab Interpretation INV (n=2588) CON (n=2591) Stress imaging (%) 75 76 Exercise tolerance test (ETT) (%) 25 24 Severe 53% 55% Moderate 34% 32% Mild/None 12% Uninterpretable 1% 1% Stress Test Modality Baseline Inducible Ischemia* *Only severe qualified by ETT Scientific. Sessions. org#AHA 19

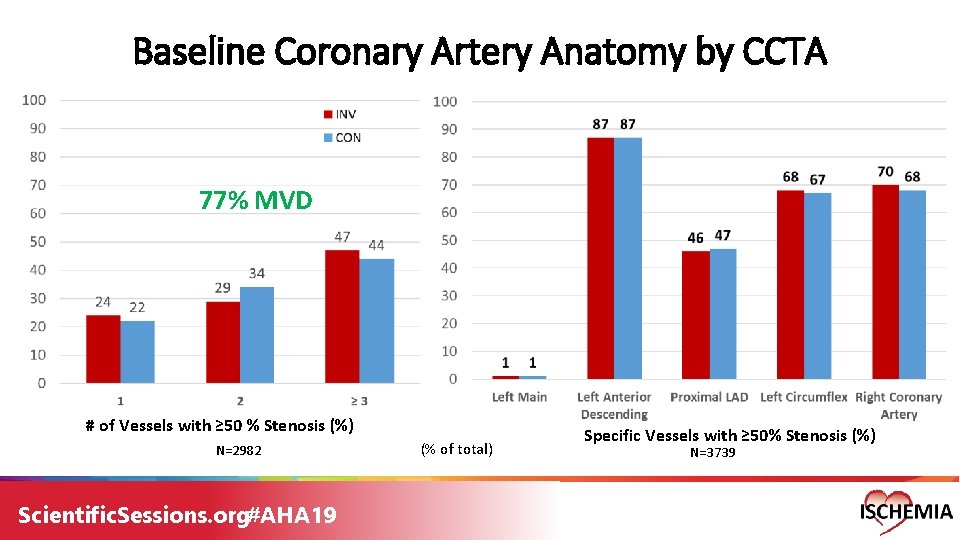
Baseline Coronary Artery Anatomy by CCTA 77% MVD # of Vessels with ≥ 50 % Stenosis (%) N=2982 Scientific. Sessions. org#AHA 19 (% of total) Specific Vessels with ≥ 50% Stenosis (%) N=3739

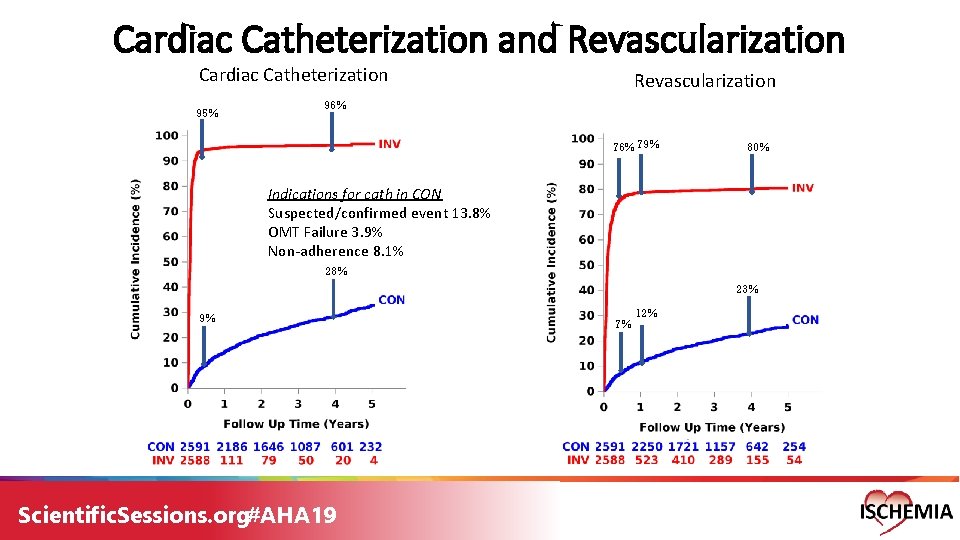
Cardiac Catheterization and Revascularization Cardiac Catheterization 95% Revascularization 96% 79% 80% Indications for cath in CON Suspected/confirmed event 13. 8% OMT Failure 3. 9% Non-adherence 8. 1% 28% 23% 9% Scientific. Sessions. org#AHA 19 7% 12%

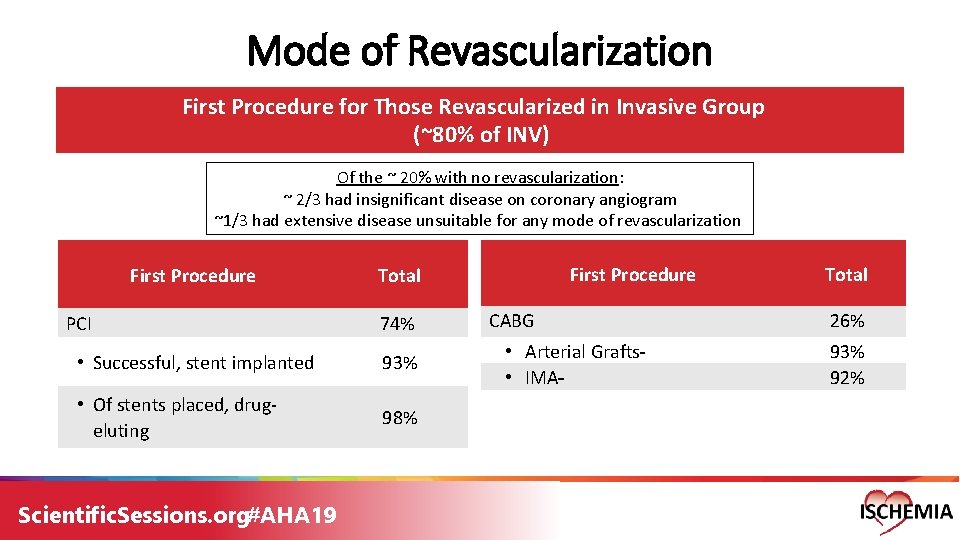
Mode of Revascularization First Procedure for Those Revascularized in Invasive Group (~80% of INV) Of the ~ 20% with no revascularization: ~ 2/3 had insignificant disease on coronary angiogram ~1/3 had extensive disease unsuitable for any mode of revascularization First Procedure PCI 74% • Successful, stent implanted 93% • Of stents placed, drug- eluting 98% Scientific. Sessions. org#AHA 19 First Procedure Total CABG • Arterial Grafts • IMA Total 26% 93% 92%

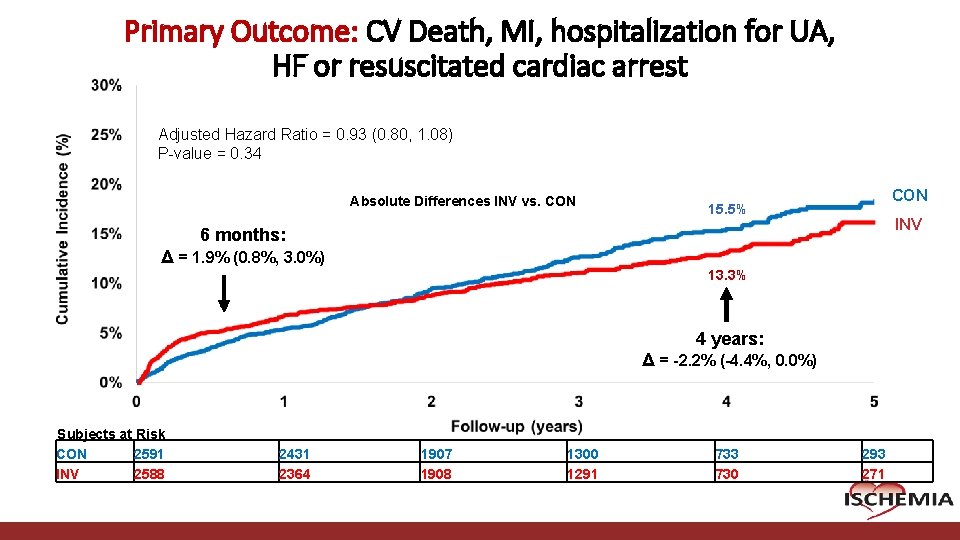
Primary Outcome: CV Death, MI, hospitalization for UA, HF or resuscitated cardiac arrest Adjusted Hazard Ratio = 0. 93 (0. 80, 1. 08) P-value = 0. 34 Absolute Differences INV vs. CON 15. 5% INV 6 months: Δ = 1. 9% (0. 8%, 3. 0%) 13. 3% 4 years: Δ = -2. 2% (-4. 4%, 0. 0%) Subjects at Risk CON 2591 INV 2588 Cardiovascular Clinical Research Center 2431 2364 1907 1908 1300 1291 733 730 293 271

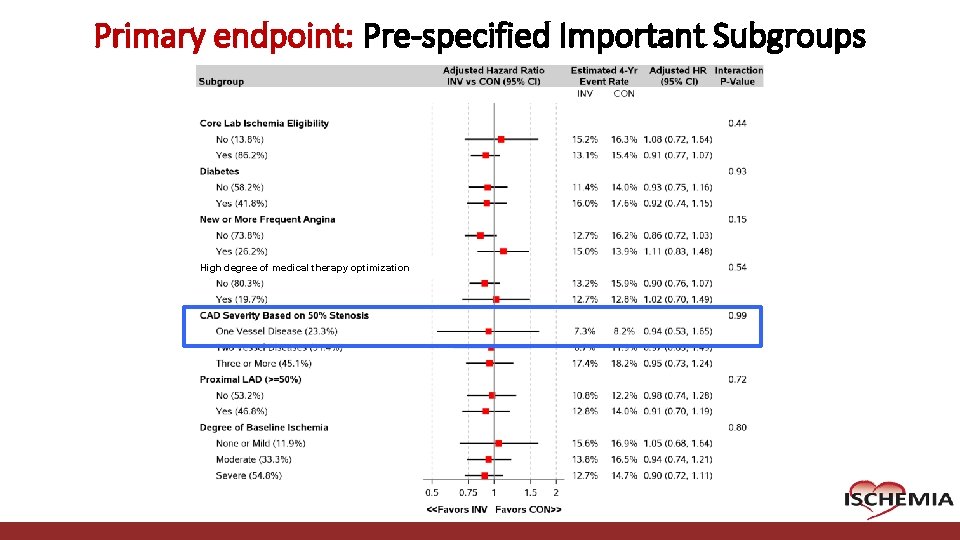
Primary endpoint: Pre-specified Important Subgroups High degree of medical therapy optimization Cardiovascular Clinical Research Center

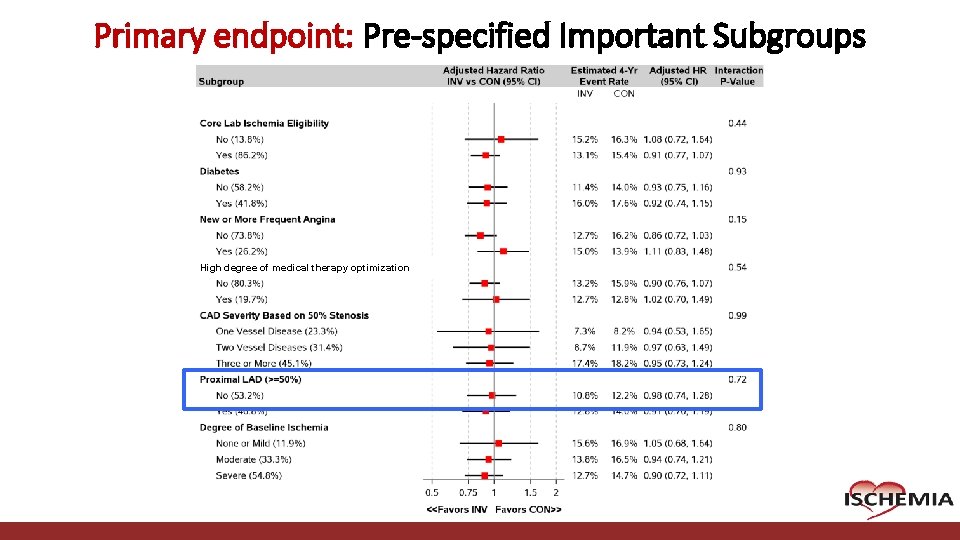
Primary endpoint: Pre-specified Important Subgroups High degree of medical therapy optimization Cardiovascular Clinical Research Center

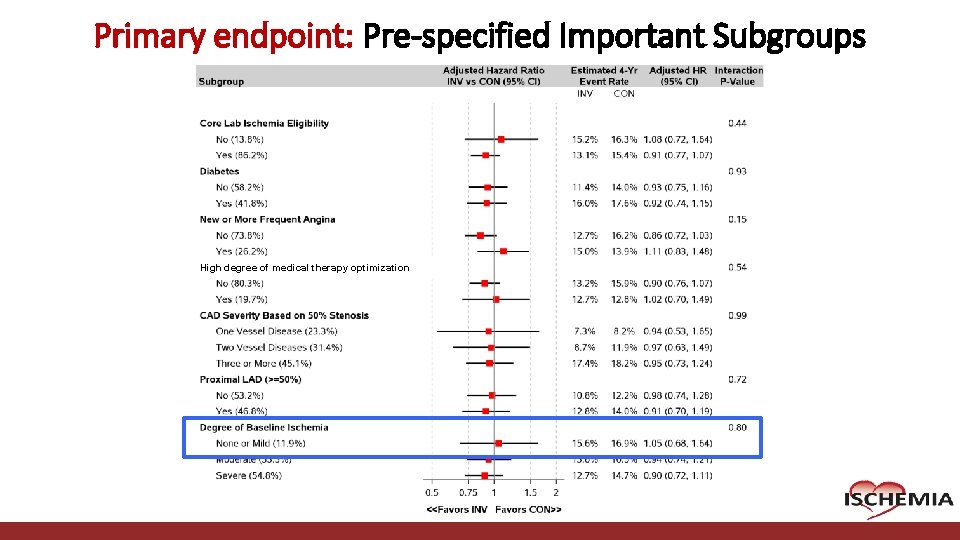
Primary endpoint: Pre-specified Important Subgroups High degree of medical therapy optimization Cardiovascular Clinical Research Center

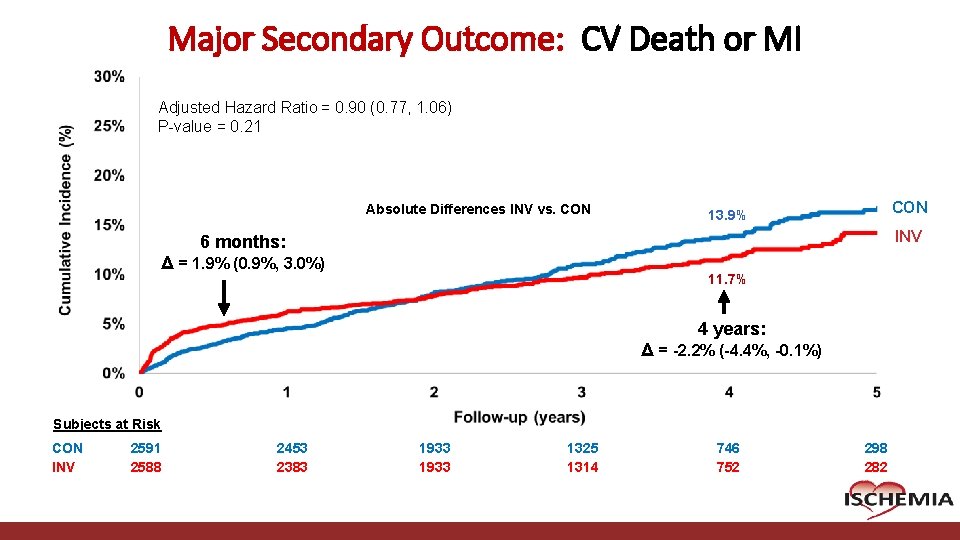
Major Secondary Outcome: CV Death or MI Adjusted Hazard Ratio = 0. 90 (0. 77, 1. 06) P-value = 0. 21 Absolute Differences INV vs. CON 13. 9% INV 6 months: Δ = 1. 9% (0. 9%, 3. 0%) 11. 7% 4 years: Δ = -2. 2% (-4. 4%, -0. 1%) Subjects at Risk CON INV 2591 2588 Cardiovascular Clinical Research Center 2453 2383 1933 1325 1314 746 752 298 282

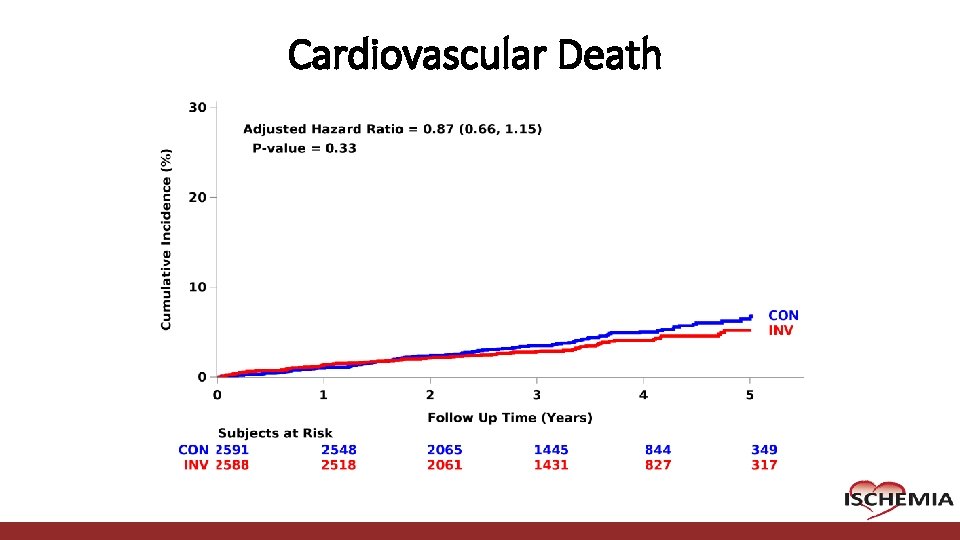
Cardiovascular Death Cardiovascular Clinical Research Center

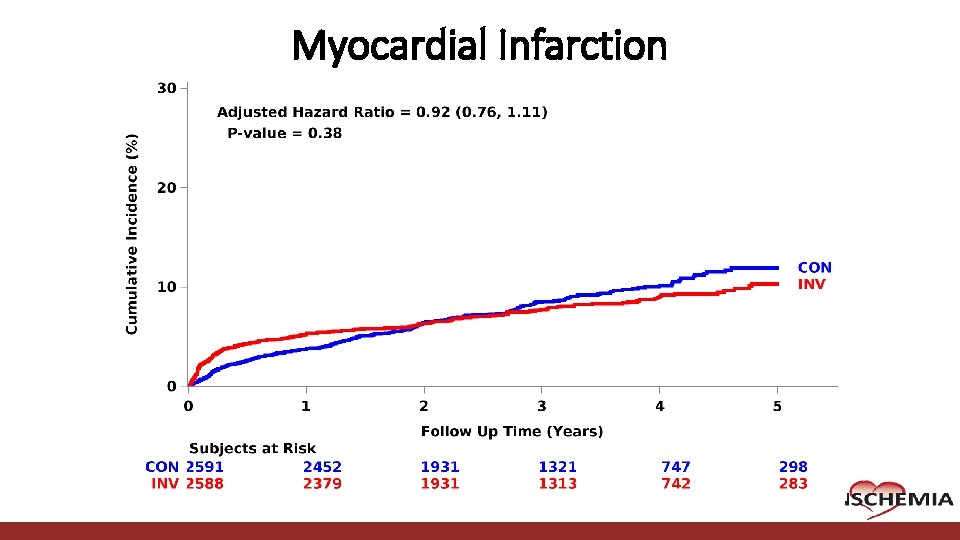
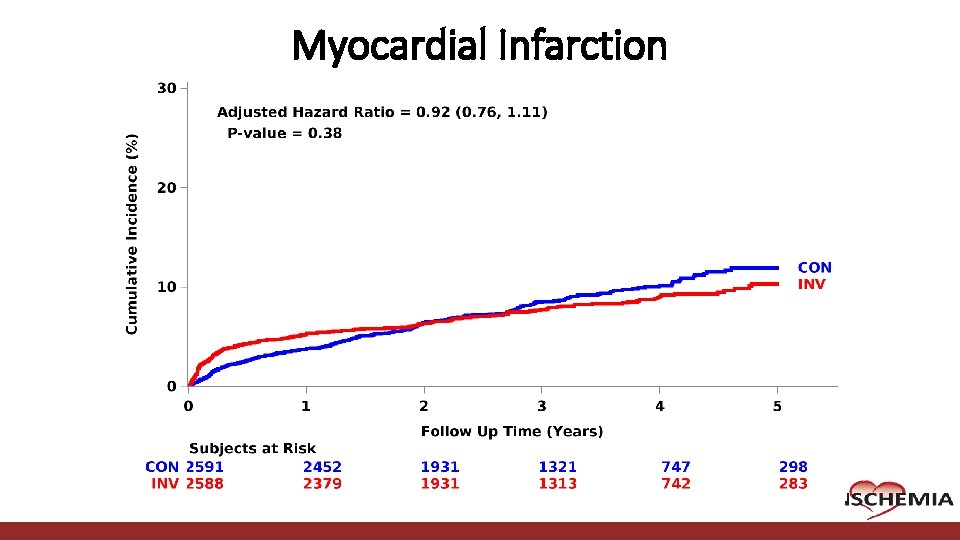
Myocardial Infarction Cardiovascular Clinical Research Center

Myocardial Infarction Cardiovascular Clinical Research Center

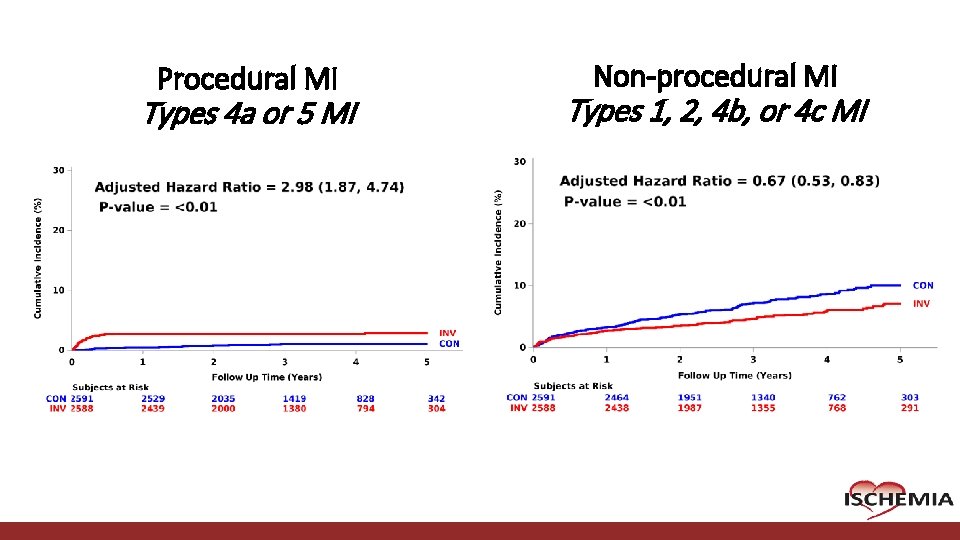
Procedural MI Types 4 a or 5 MI Cardiovascular Clinical Research Center Non-procedural MI Types 1, 2, 4 b, or 4 c MI

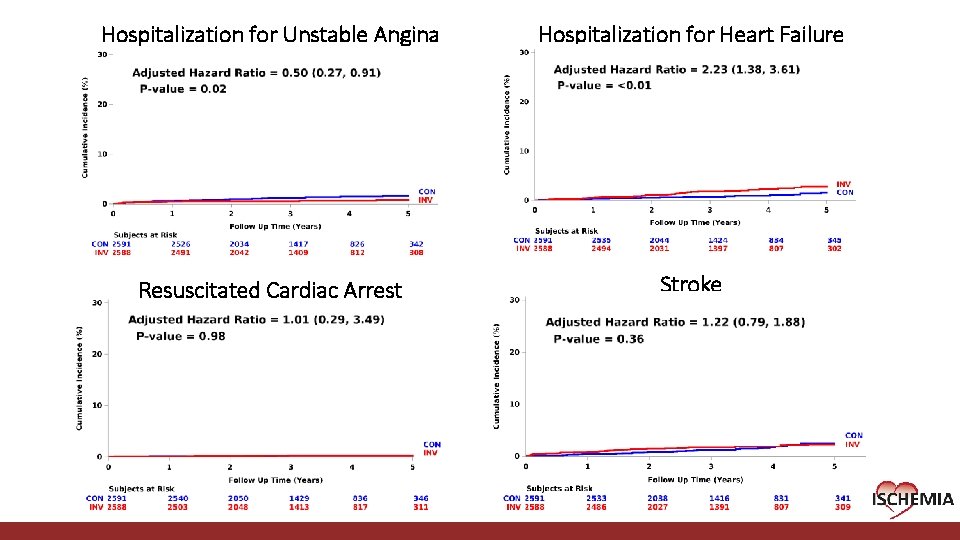
Hospitalization for Unstable Angina Hospitalization for Heart Failure Resuscitated Cardiac Arrest Stroke Cardiovascular Clinical Research Center

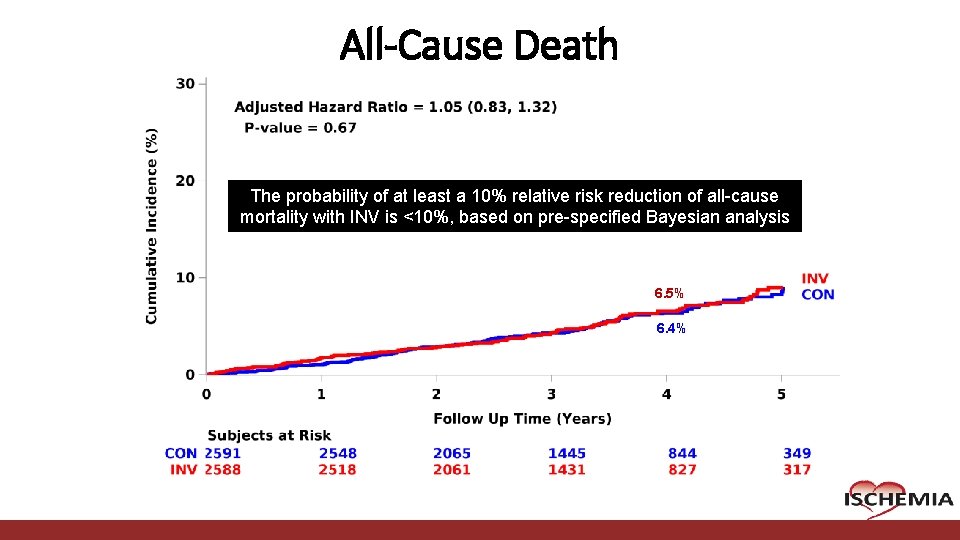
All-Cause Death The probability of at least a 10% relative risk reduction of all-cause mortality with INV is <10%, based on pre-specified Bayesian analysis 6. 5% 6. 4% Cardiovascular Clinical Research Center

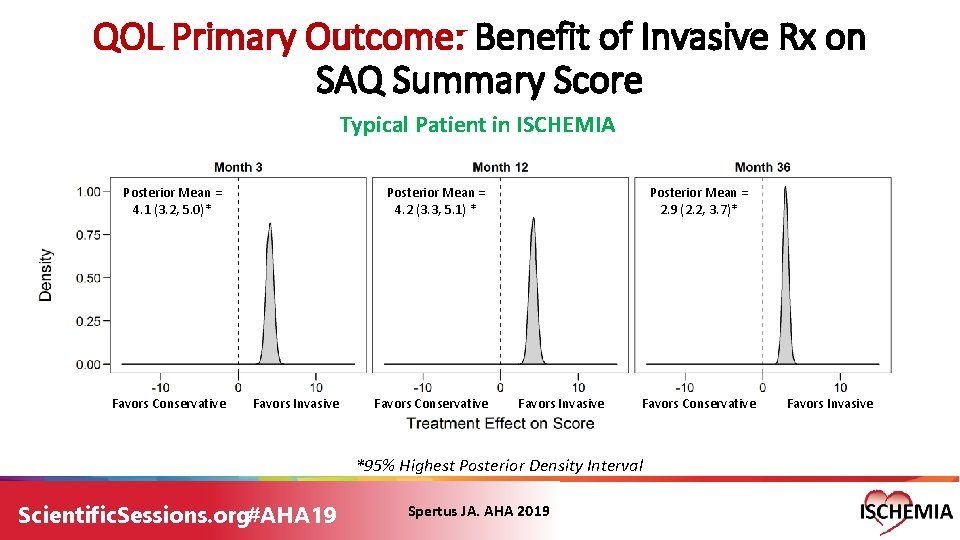
QOL Primary Outcome: Benefit of Invasive Rx on SAQ Summary Score Typical Patient in ISCHEMIA Posterior Mean = 4. 1 (3. 2, 5. 0)* Favors Conservative Posterior Mean = 4. 2 (3. 3, 5. 1) * Favors Invasive Favors Conservative Posterior Mean = 2. 9 (2. 2, 3. 7)* Favors Invasive Favors Conservative *95% Highest Posterior Density Interval Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019 Favors Invasive

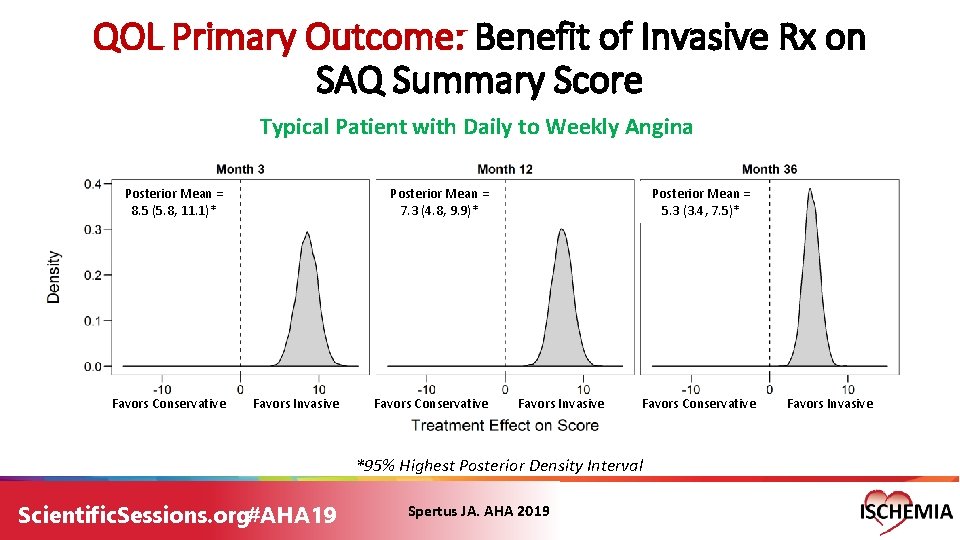
QOL Primary Outcome: Benefit of Invasive Rx on SAQ Summary Score Typical Patient with Daily to Weekly Angina Posterior Mean = 8. 5 (5. 8, 11. 1)* Favors Conservative Posterior Mean = 7. 3 (4. 8, 9. 9)* Favors Invasive Favors Conservative Posterior Mean = 5. 3 (3. 4, 7. 5)* Favors Invasive Favors Conservative *95% Highest Posterior Density Interval Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019 Favors Invasive

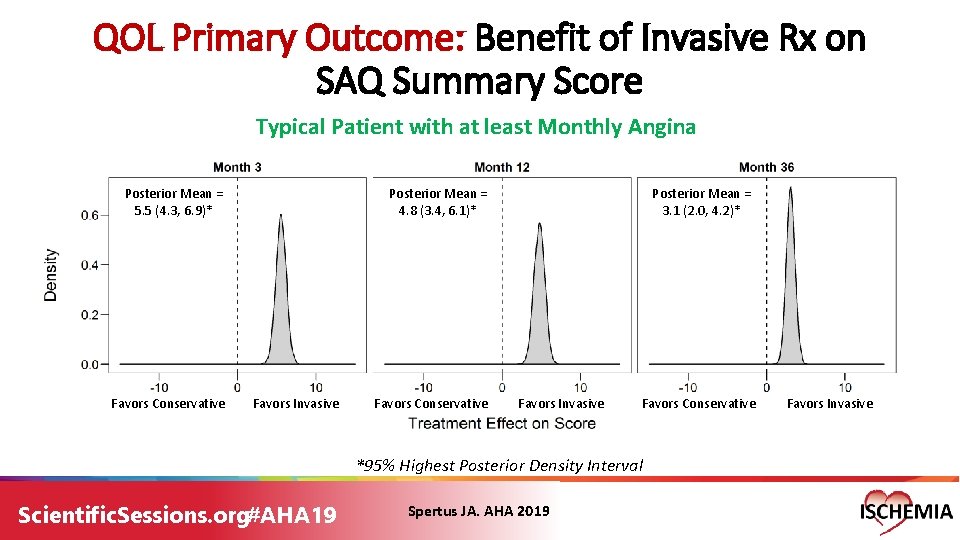
QOL Primary Outcome: Benefit of Invasive Rx on SAQ Summary Score Typical Patient with at least Monthly Angina Posterior Mean = 5. 5 (4. 3, 6. 9)* Favors Conservative Posterior Mean = 4. 8 (3. 4, 6. 1)* Favors Invasive Favors Conservative Posterior Mean = 3. 1 (2. 0, 4. 2)* Favors Invasive Favors Conservative *95% Highest Posterior Density Interval Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019 Favors Invasive

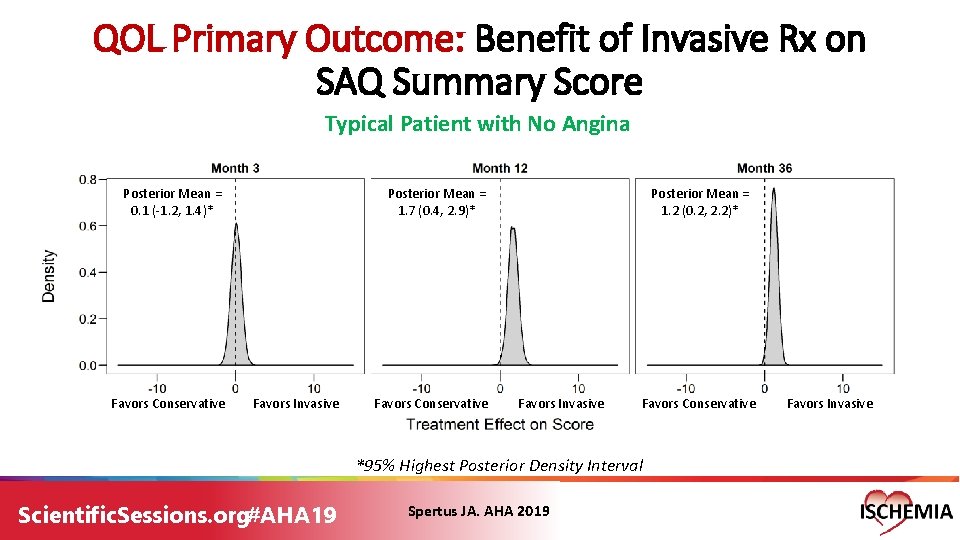
QOL Primary Outcome: Benefit of Invasive Rx on SAQ Summary Score Typical Patient with No Angina Posterior Mean = 0. 1 (-1. 2, 1. 4)* Favors Conservative Posterior Mean = 1. 7 (0. 4, 2. 9)* Favors Invasive Favors Conservative Posterior Mean = 1. 2 (0. 2, 2. 2)* Favors Invasive Favors Conservative *95% Highest Posterior Density Interval Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019 Favors Invasive

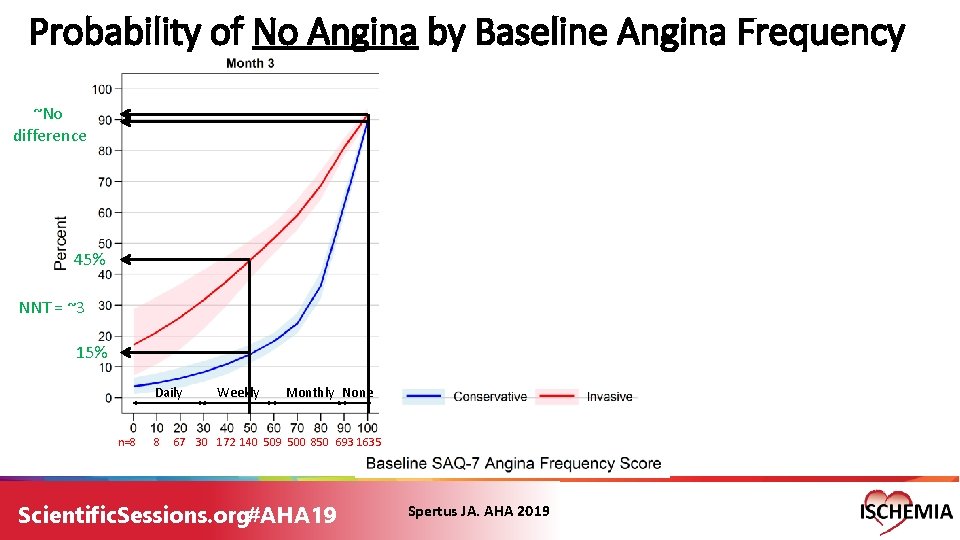
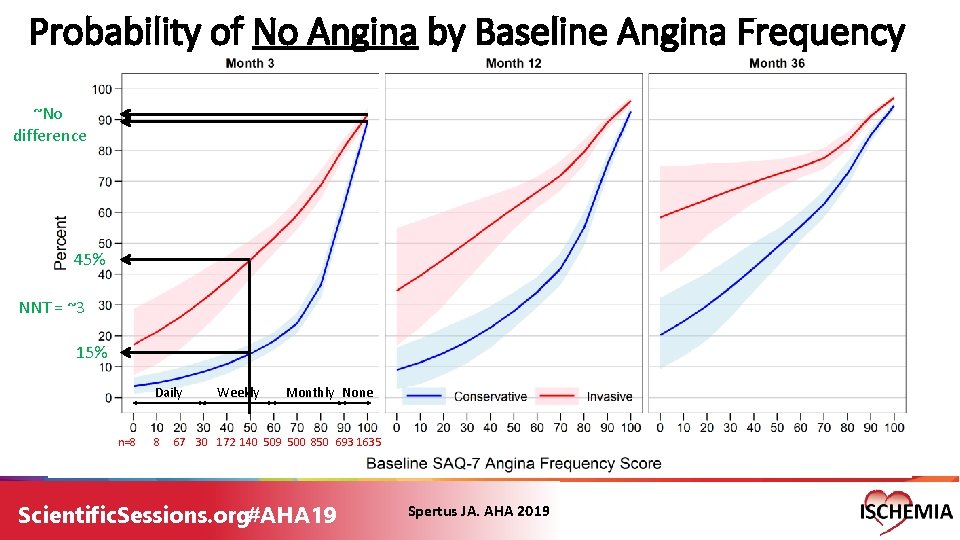
Probability of No Angina by Baseline Angina Frequency ~No difference 45% NNT = ~3 15% Daily n=8 8 Weekly Monthly None 67 30 172 140 509 500 850 693 1635 Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019

Probability of No Angina by Baseline Angina Frequency ~No difference 45% NNT = ~3 15% Daily n=8 8 Weekly Monthly None 67 30 172 140 509 500 850 693 1635 Scientific. Sessions. org#AHA 19 Spertus JA. AHA 2019

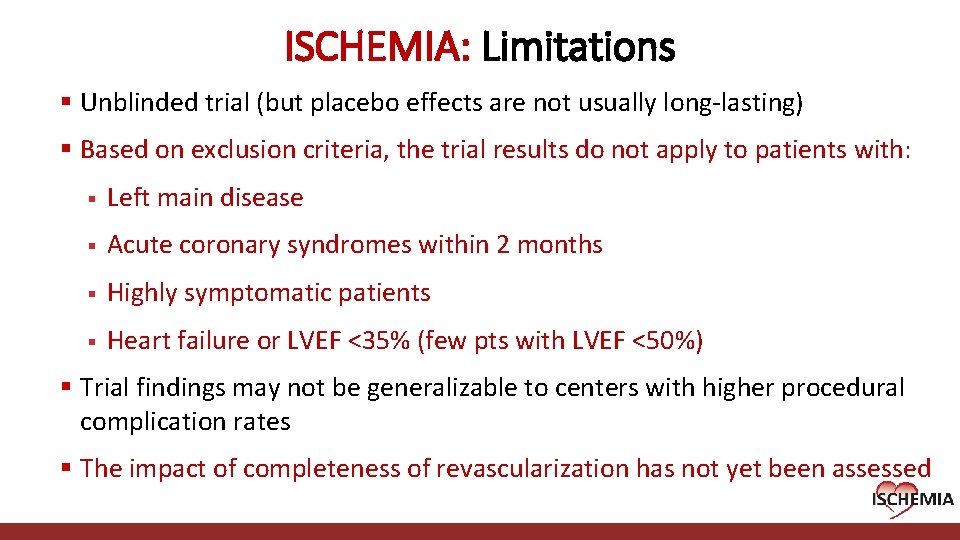
ISCHEMIA: Limitations § Unblinded trial (but placebo effects are not usually long-lasting) § Based on exclusion criteria, the trial results do not apply to patients with: § Left main disease § Acute coronary syndromes within 2 months § Highly symptomatic patients § Heart failure or LVEF <35% (few pts with LVEF <50%) § Trial findings may not be generalizable to centers with higher procedural complication rates § The impact of completeness of revascularization has not yet been assessed Cardiovascular Clinical Research Center

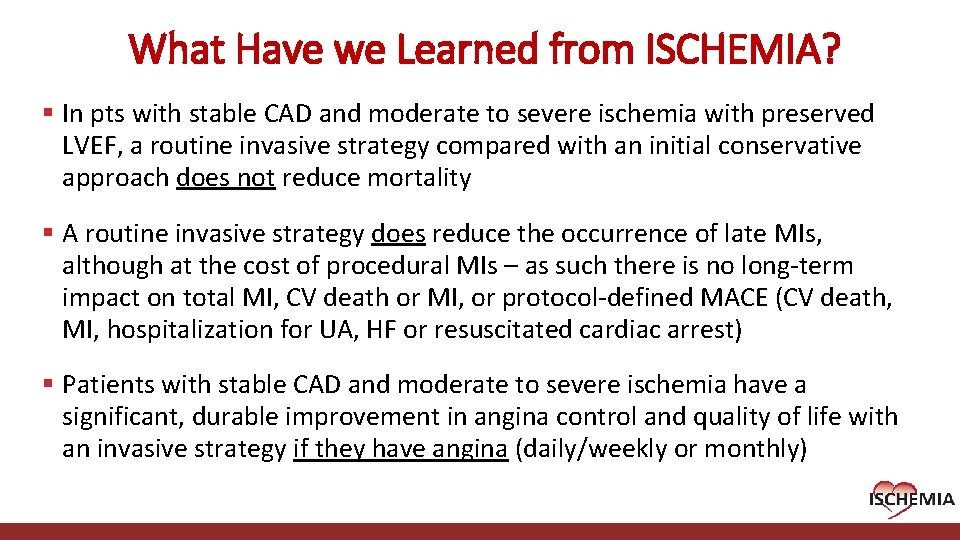
What Have we Learned from ISCHEMIA? § In pts with stable CAD and moderate to severe ischemia with preserved LVEF, a routine invasive strategy compared with an initial conservative approach does not reduce mortality § A routine invasive strategy does reduce the occurrence of late MIs, although at the cost of procedural MIs – as such there is no long-term impact on total MI, CV death or MI, or protocol-defined MACE (CV death, MI, hospitalization for UA, HF or resuscitated cardiac arrest) § Patients with stable CAD and moderate to severe ischemia have a significant, durable improvement in angina control and quality of life with an invasive strategy if they have angina (daily/weekly or monthly) Cardiovascular Clinical Research Center

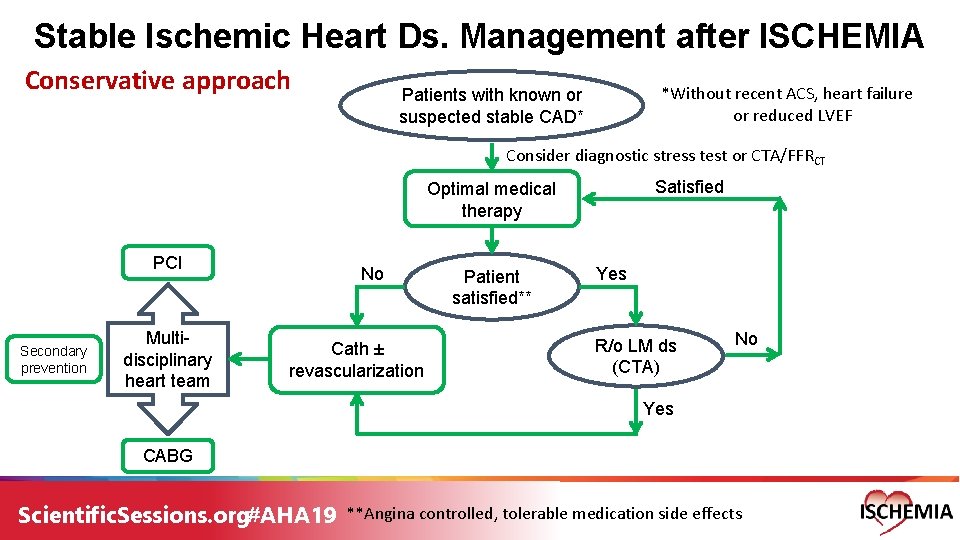
Stable Ischemic Heart Ds. Management after ISCHEMIA Conservative approach *Without recent ACS, heart failure or reduced LVEF Patients with known or suspected stable CAD* Consider diagnostic stress test or CTA/FFRCT Satisfied Optimal medical therapy PCI Secondary prevention Multidisciplinary heart team No Cath ± revascularization Patient satisfied** Yes R/o LM ds (CTA) No Yes CABG Scientific. Sessions. org#AHA 19 **Angina controlled, tolerable medication side effects

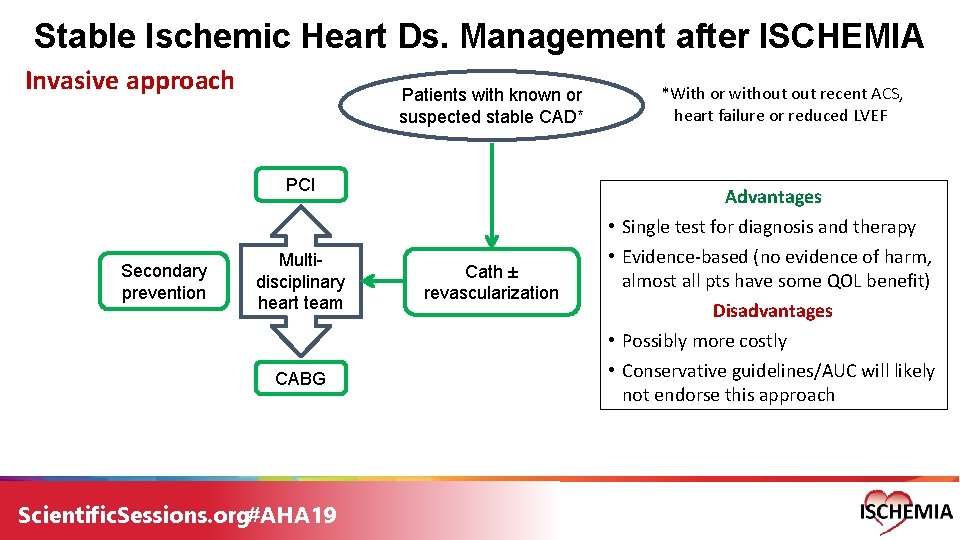
Stable Ischemic Heart Ds. Management after ISCHEMIA Invasive approach Patients with known or suspected stable CAD* PCI Secondary prevention Multidisciplinary heart team CABG Scientific. Sessions. org#AHA 19 *With or without recent ACS, heart failure or reduced LVEF Advantages Cath ± revascularization • Single test for diagnosis and therapy • Evidence-based (no evidence of harm, almost all pts have some QOL benefit) Disadvantages • Possibly more costly • Conservative guidelines/AUC will likely not endorse this approach