CREATING THE ENDOVASCULAR AUTOLOGOUS ARTERIOVENOUS FISTULA ENDOAVF IN








- Slides: 48

CREATING THE ENDOVASCULAR AUTOLOGOUS ARTERIOVENOUS FISTULA (ENDOAVF) IN THE UPPER LIMB Athanasios D. Giannoukas, MSc(Lond. ), MD, Ph. D(Lond. ), FEBVS Professor of Vascular Surgery Dean of Medical School University of Thessaly Chairman, Department of Vascular Surgery University Hospital of Larissa, Greece

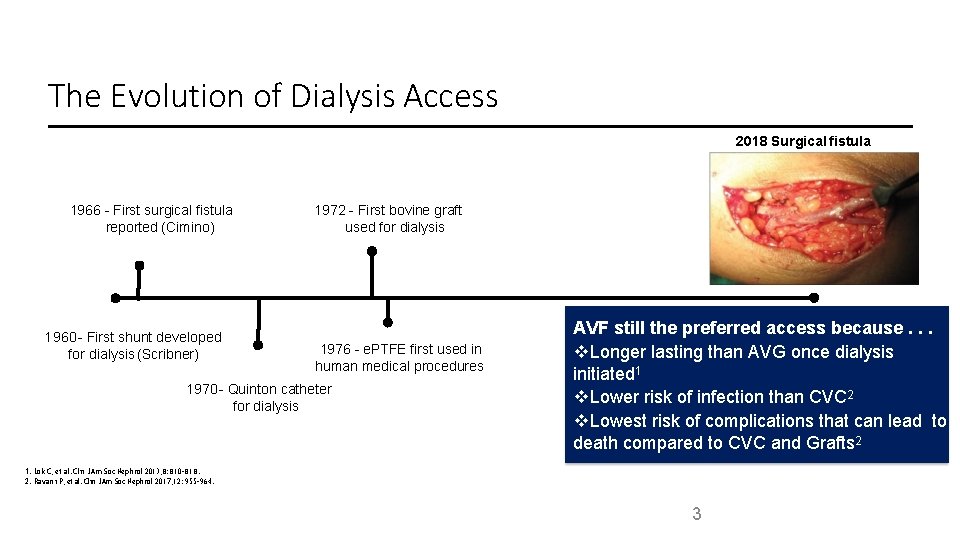
The Evolution of Dialysis Access 2018 Surgical fistula 1966 - First surgical fistula reported (Cimino) 1960 - First shunt developed for dialysis (Scribner) 1972 - First bovine graft used for dialysis 1976 - e. PTFE first used in human medical procedures 1970 - Quinton catheter for dialysis AVF still the preferred access because. . . Longer lasting than AVG once dialysis initiated 1 Lower risk of infection than CVC 2 Lowest risk of complications that can lead to death compared to CVC and Grafts 2 1. Lok C, et al. Clin J Am Soc Nephrol 2013; 8: 810– 818. 2. Ravani P, et al. Clin J Am Soc Nephrol 2017; 12: 955– 964. 3

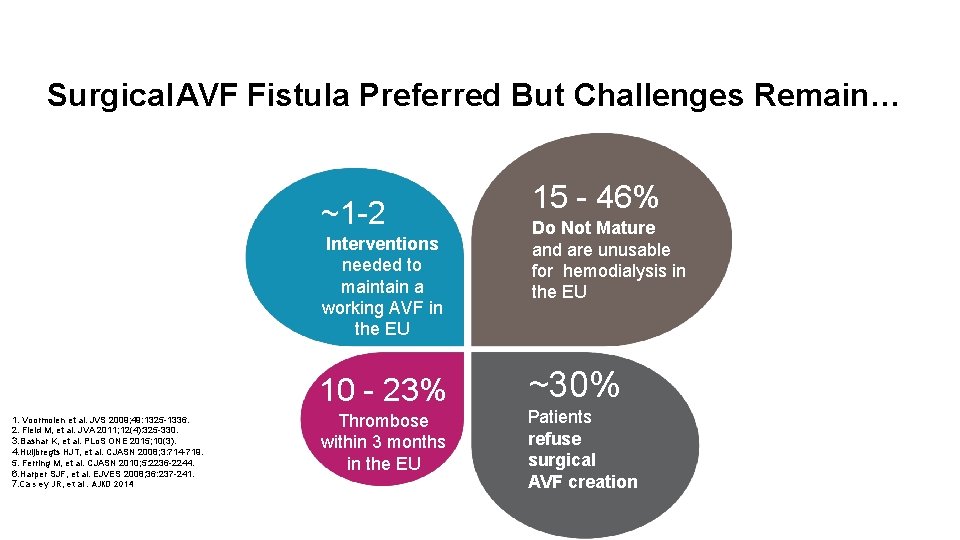
Surgical AVF Fistula Preferred But Challenges Remain… ~1 -2 Interventions needed to maintain a working AVF in the EU 10 - 23% 1. Voormolen et al. JVS 2009; 49: 1325 -1336. 2. Field M, et al. JVA 2011; 12(4): 325 -330. 3. Bashar K, et al. PLo. S ONE 2015; 10(3). 4. Huijbregts HJT, et al. CJASN 2008; 3: 714 -719. 5. Ferring M, et al. CJASN 2010; 5: 2236 -2244. 6. Harper SJF, et al. EJVES 2008; 36: 237 -241. 7. Ca s ey JR, et al. AJKD 2014 Thrombose within 3 months in the EU 15 - 46% Do Not Mature and are unusable for hemodialysis in the EU ~30% Patients refuse surgical AVF creation

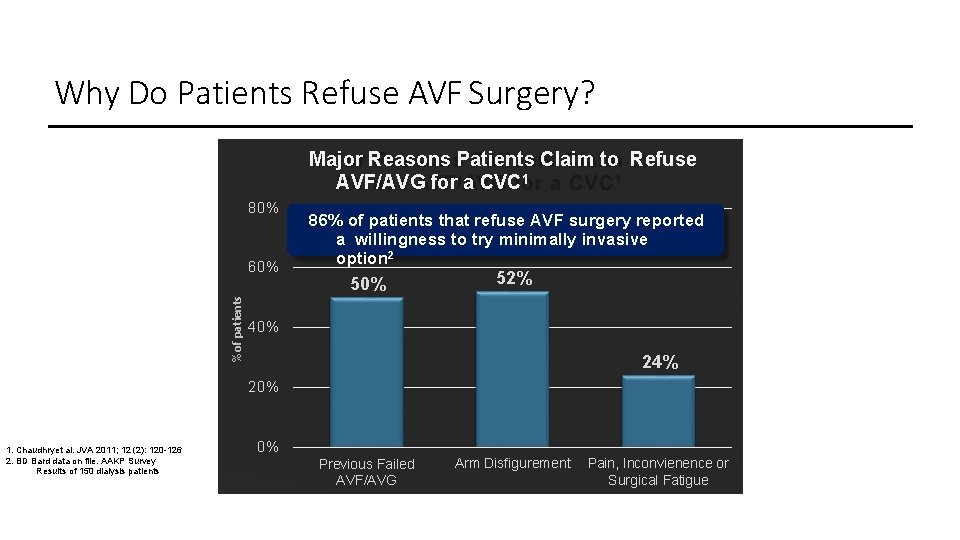
Why Do Patients Refuse AVF Surgery? Major Reasons Patients Claim to Refuse AVF/AVG for a CVC 1 80% % of patients 60% 86% of patients that refuse AVF surgery reported a willingness to try minimally invasive option 2 50% 52% 40% 24% 20% 1. Chaudhryet al. JVA 2011; 12 (2): 120 -126 2. BD Bard data on file. AAKP Survey Results of 150 dialysis patients 0% Previous Failed AVF/AVG Arm Disfigurement Pain, Inconvienence or Surgical Fatigue

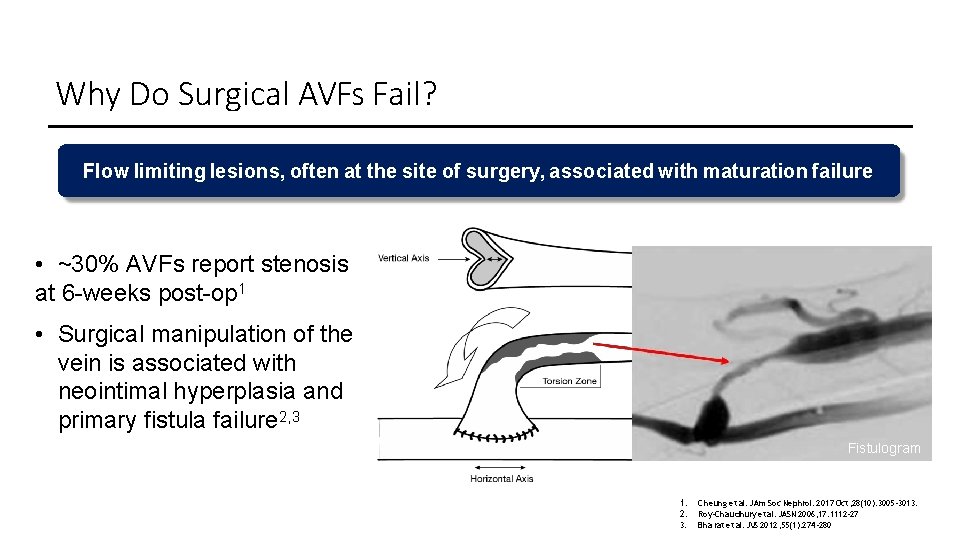
Why Do Surgical AVFs Fail? Flow limiting lesions, often at the site of surgery, associated with maturation failure • ~30% AVFs report stenosis at 6 -weeks post-op 1 • Surgical manipulation of the vein is associated with neointimal hyperplasia and primary fistula failure 2, 3 Fistulogram 1. 2. 3. Cheung et al. JAm Soc Nephrol. 2017 Oct; 28(10): 3005 -3013. Roy-Cha udhury et al. JASN 2006; 17: 1112 -27 Bha rat et al. JVS 2012; 55(1): 274 -280

WHY CREATE AN ENDOAVF?

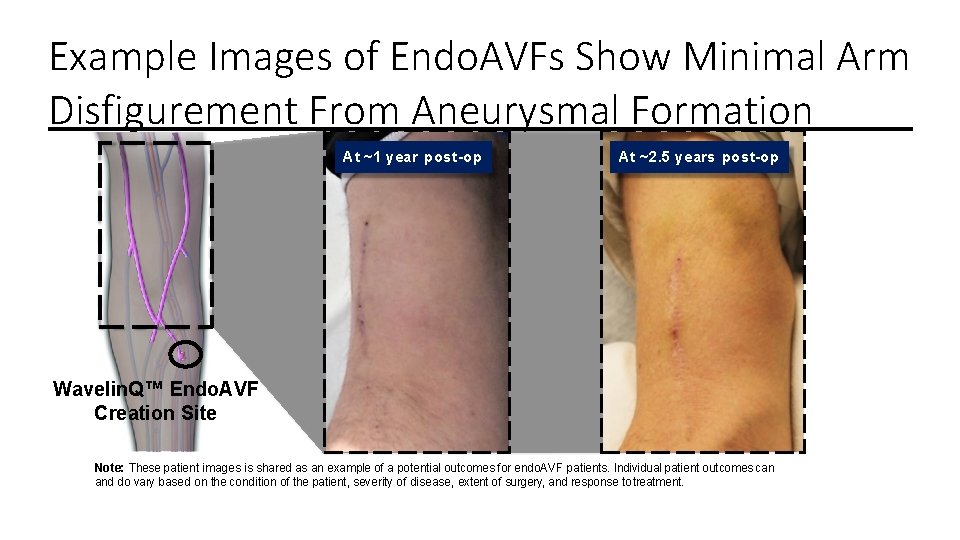
Example Images of Endo. AVFs Show Minimal Arm Disfigurement From Aneurysmal Formation At ~1 year post-op At ~2. 5 years post-op Wavelin. Q™ Endo. AVF Creation Site Note: These patient images is shared as an example of a potential outcomes for endo. AVF patients. Individual patient outcomes can and do vary based on the condition of the patient, severity of disease, extent of surgery, and response to treatment.

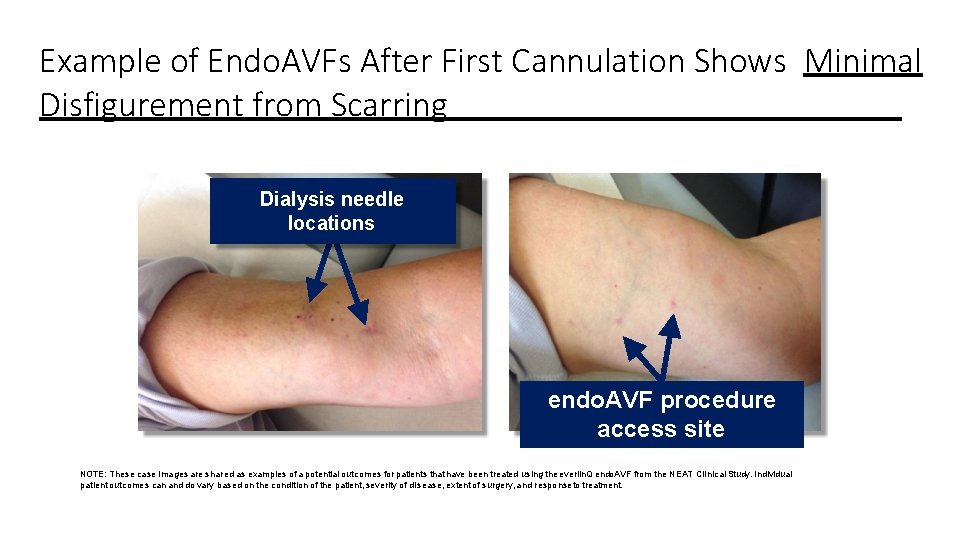
Example of Endo. AVFs After First Cannulation Shows Minimal Disfigurement from Scarring Dialysis needle locations endo. AVF procedure access site NOTE: These case images are shared as examples of a potential outcomes for patients that have been treated using the everlin. Q endo. AVF from the NEAT Clinical Study. Individual patient outcomes can and do vary based on the condition of the patient, severity of disease, extent of surgery, and responseto treatment.

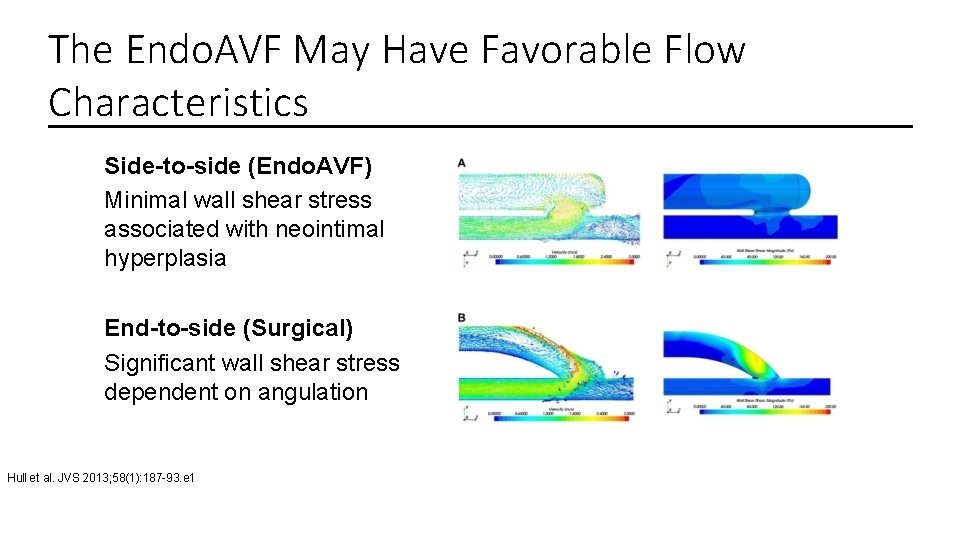
The Endo. AVF May Have Favorable Flow Characteristics Side-to-side (Endo. AVF) Minimal wall shear stress associated with neointimal hyperplasia End-to-side (Surgical) Significant wall shear stress dependent on angulation Hull et al. JVS 2013; 58(1): 187 -93. e 1

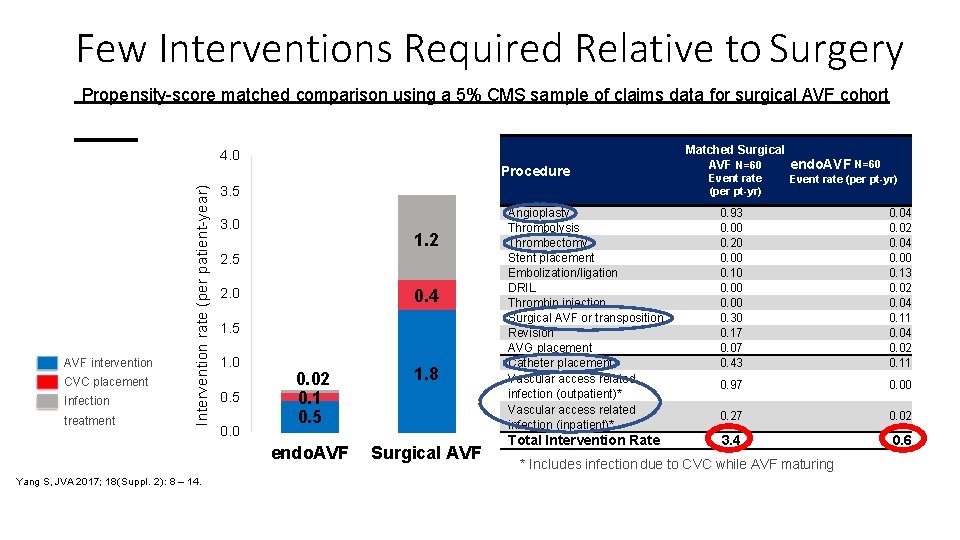
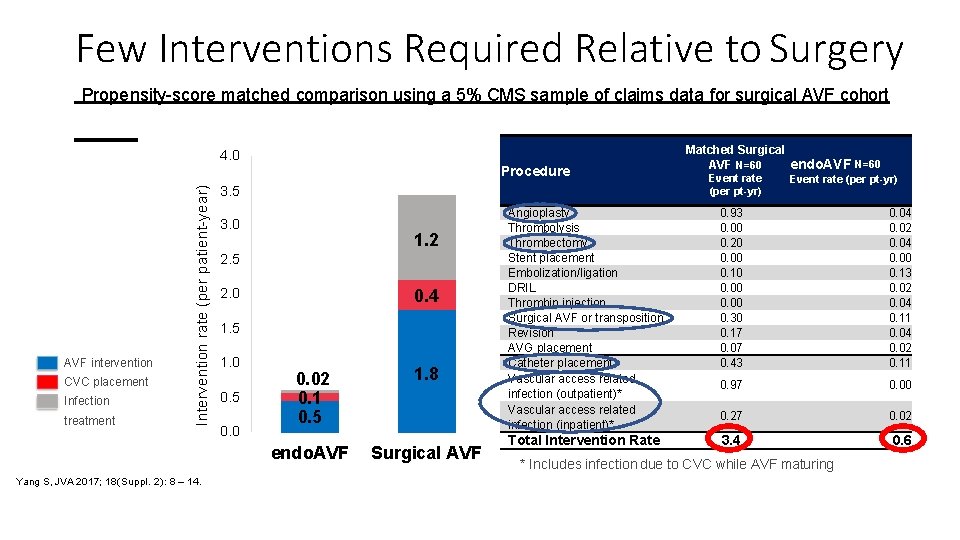
Few Interventions Required Relative to Surgery Propensity-score matched comparison using a 5% CMS sample of claims data for surgical AVF cohort 4. 0 AVF intervention CVC placement Infection treatment Intervention rate (per patient-year) Procedure Yang S, JVA 2017; 18(Suppl. 2): 8 – 14. 3. 5 3. 0 1. 2 2. 5 0. 4 2. 0 1. 5 1. 0 0. 5 0. 02 0. 1 0. 5 1. 8 endo. AVF Surgical AVF Matched Surgical AVF N=60 endo. AVF N=60 Event rate (per pt-yr) Angioplasty Thrombolysis Thrombectomy Stent placement Embolization/ligation DRIL Thrombin injection Surgical AVF or transposition Revision AVG placement Catheter placement Vascular access related infection (outpatient)* Vascular access related infection (inpatient)* 0. 93 0. 00 0. 20 0. 00 0. 10 0. 00 0. 30 0. 17 0. 07 0. 43 0. 04 0. 02 0. 04 0. 00 0. 13 0. 02 0. 04 0. 11 0. 04 0. 02 0. 11 0. 97 0. 00 0. 27 0. 02 Total Intervention Rate 3. 4 0. 6 * Includes infection due to CVC while AVF maturing

HOW IT WORKS


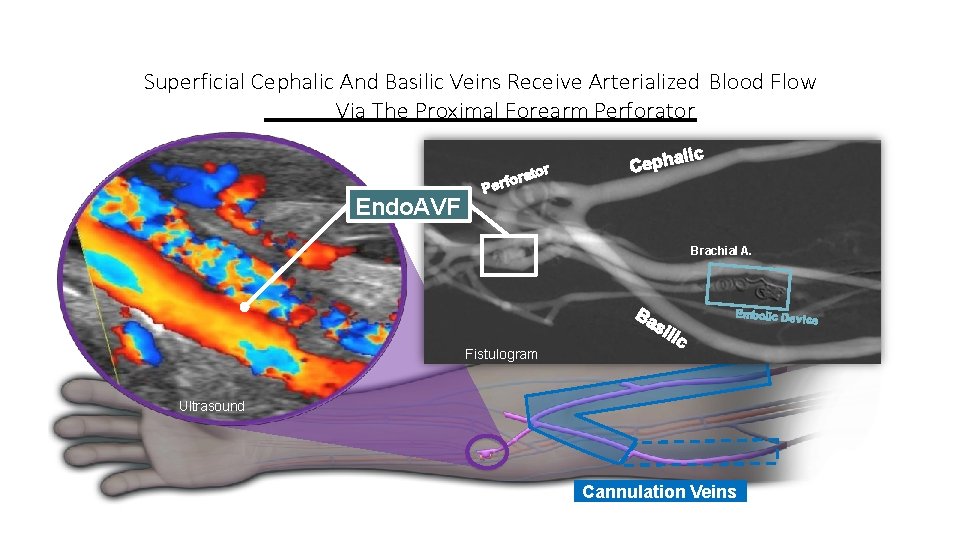
Superficial Cephalic And Basilic Veins Receive Arterialized Blood Flow Via The Proximal Forearm Perforator Endo. AVF Brachial A. Fistulogram Ultrasound Cannulation Veins

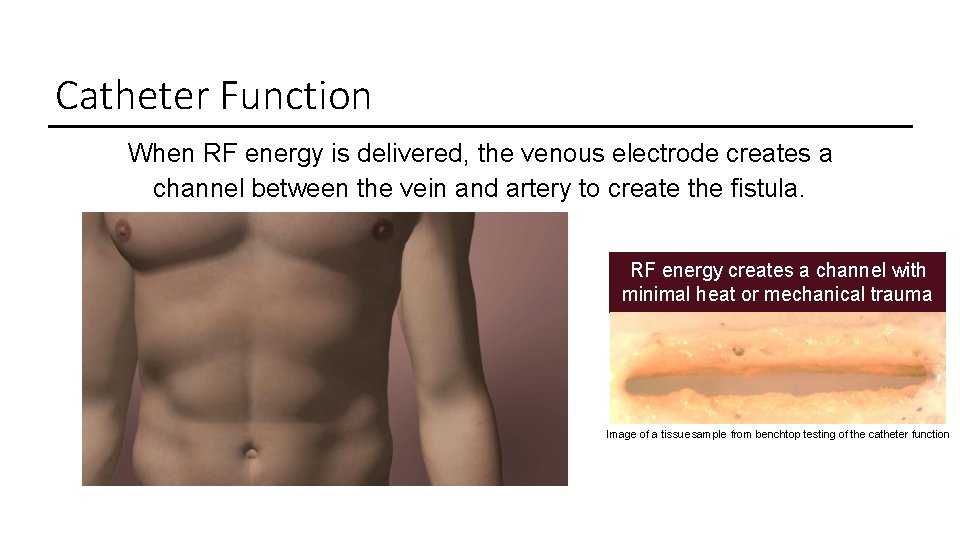
Catheter Function When RF energy is delivered, the venous electrode creates a channel between the vein and artery to create the fistula. RF energy creates a channel with minimal heat or mechanical trauma Image of a tissue sample from benchtop testing of the catheter function

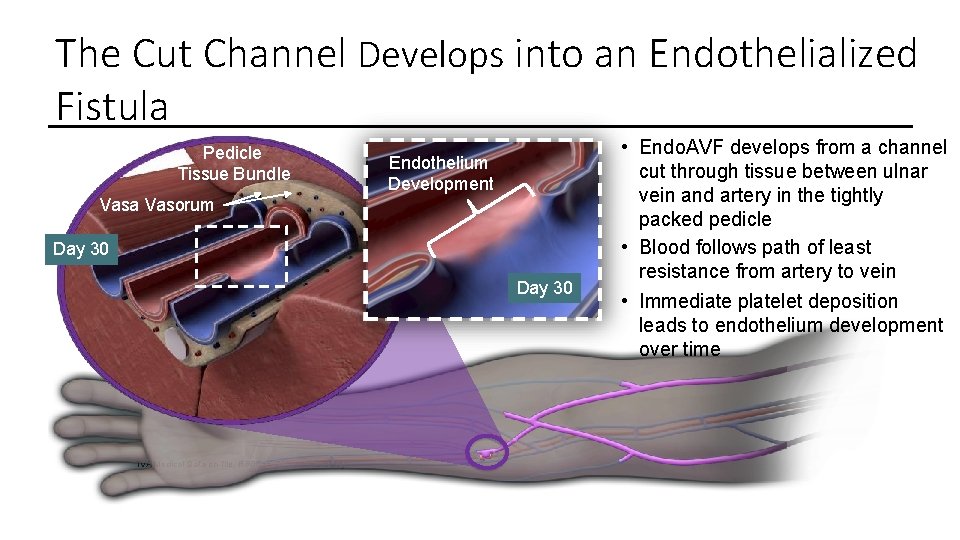
The Cut Channel Develops into an Endothelialized Fistula Pedicle Tissue Bundle Vasa Vasorum Endothelium Platelet Development Deposition Day 30 0 Endothelium. Day Development TVA Medical Data on file. RR 0055 GLP Animal Study. • Endo. AVF develops from a channel cut through tissue between ulnar vein and artery in the tightly packed pedicle • Blood follows path of least resistance from artery to vein • Immediate platelet deposition leads to endothelium development over time

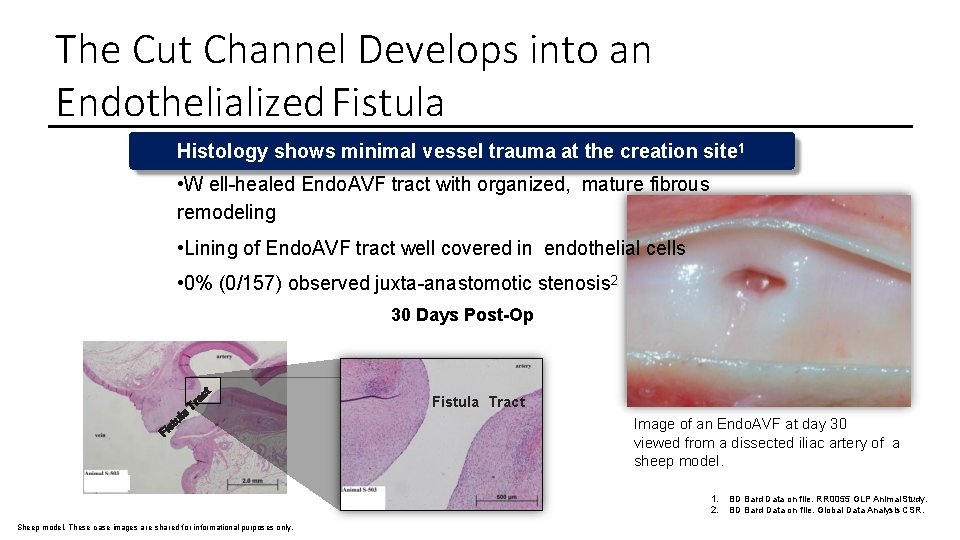
The Cut Channel Develops into an Endothelialized Fistula Histology shows minimal vessel trauma at the creation site 1 • W ell-healed Endo. AVF tract with organized, mature fibrous remodeling • Lining of Endo. AVF tract well covered in endothelial cells • 0% (0/157) observed juxta-anastomotic stenosis 2 30 Days Post-Op Fistula Tract Image of an Endo. AVF at day 30 viewed from a dissected iliac artery of a sheep model. 1. 2. Sheep model. These case images are shared for informational purposes only. BD Bard Data on file. RR 0055 GLP Animal Study. BD Bard Data on file. Global Data Analysis CSR.

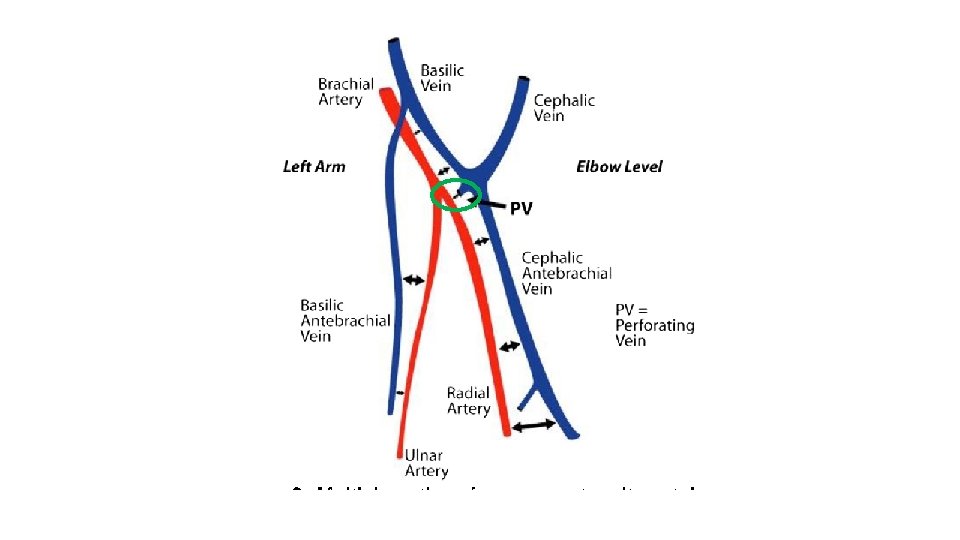
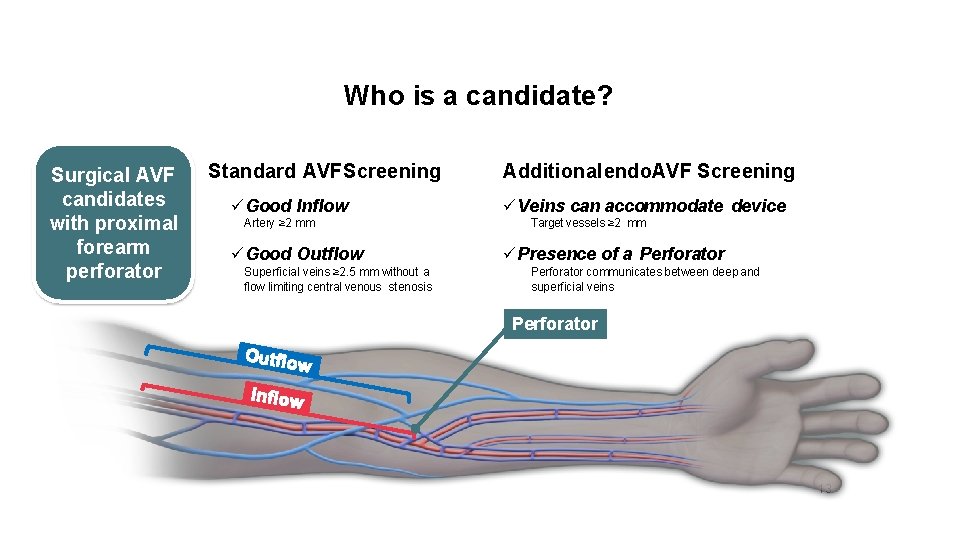
Who is a candidate? Surgical AVF candidates with proximal forearm perforator Standard AVF Screening Good Inflow Artery ≥ 2 mm Good Outflow Superficial veins ≥ 2. 5 mm without a flow limiting central venous stenosis Additional endo. AVF Screening Veins can accommodate device Target vessels ≥ 2 mm Presence of a Perforator communicates between deep and superficial veins Perforator 13

Disadvantages • • • Anatomical restrictions: nontortuous target vessels ≥ 2 mm, the presence of a ≥ 2 mm perforator vein, patent cephalic and basilic draining veins in the target upper extremity and candidates for a surgical radial-cephalic AVF were excluded, as were those with central outflow occlusion • Cost

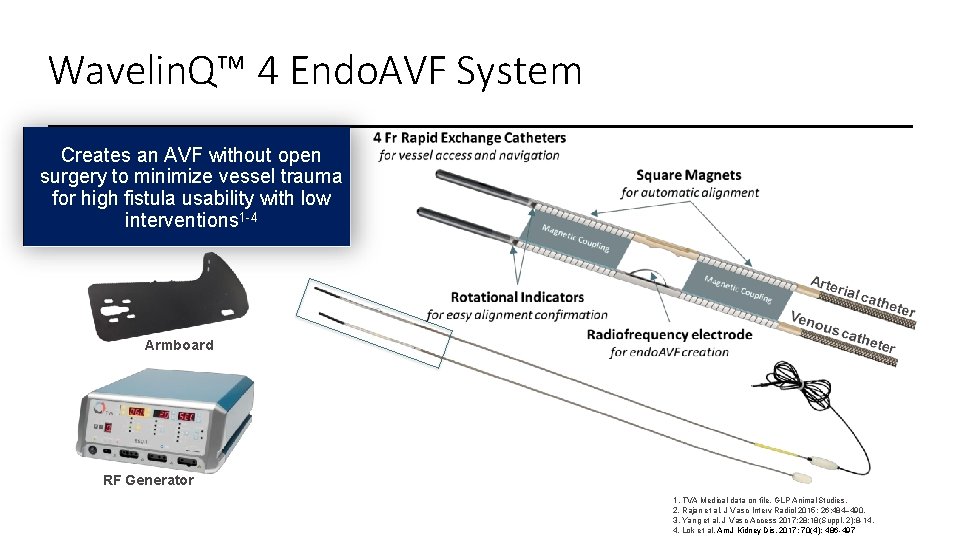
Wavelin. Q™ 4 Endo. AVF System Creates an AVF without open surgery to minimize vessel trauma for high fistula usability with low interventions 1 -4 Armboard RF Generator 1. TVA Medical data on file. GLP Animal Studies. 2. Rajan et al. J Vasc Interv Radiol 2015; 26: 484– 490. 3. Yang et al. J Vasc Access 2017; 28; 18(Suppl. 2): 8 -14. 4. Lok et al. Am J Kidney Dis. 2017; 70(4): 486 -497

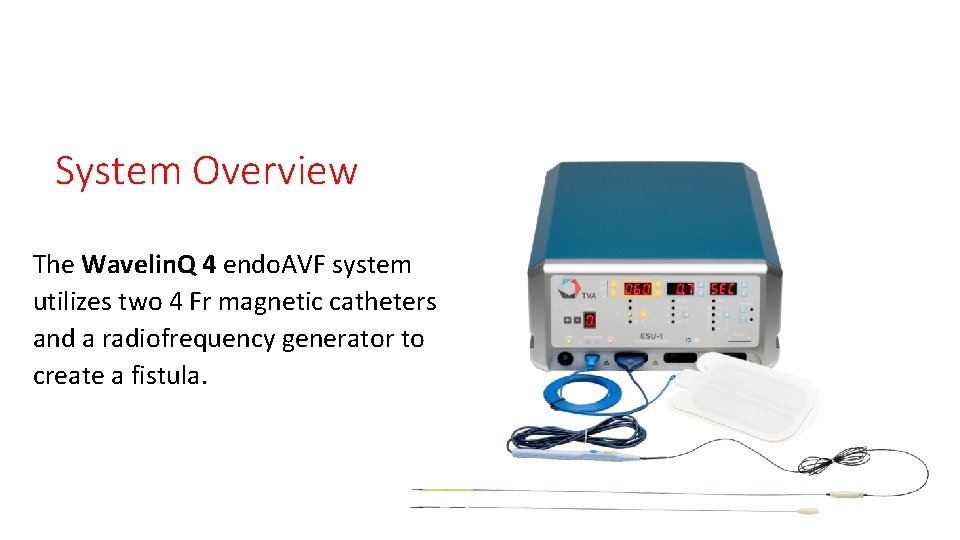
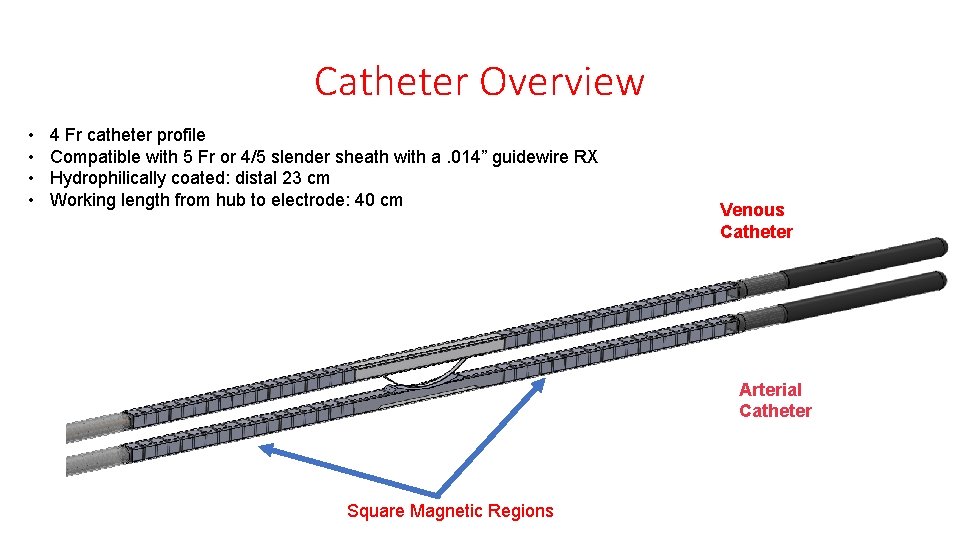
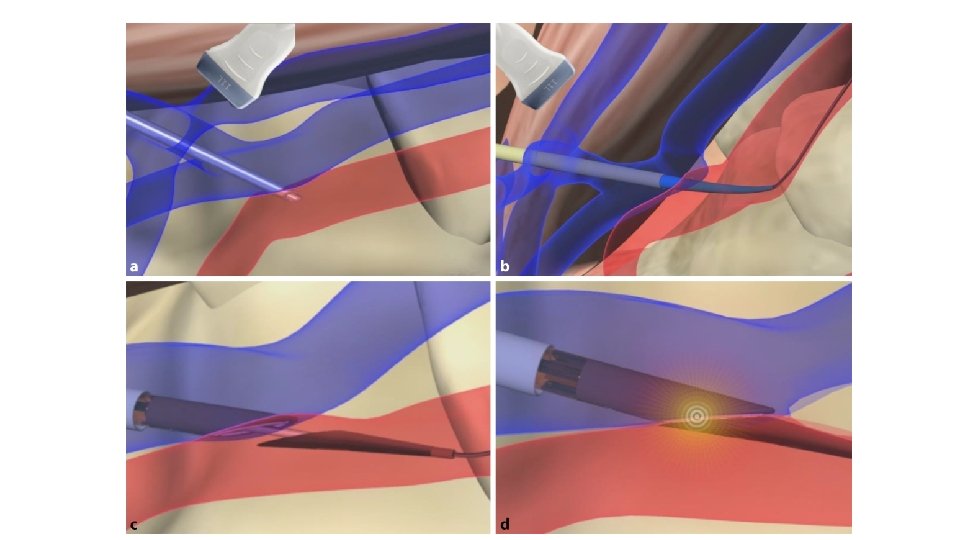
System Overview The Wavelin. Q 4 endo. AVF system utilizes two 4 Fr magnetic catheters and a radiofrequency generator to create a fistula.

Vascular access Venous access: From the brachial vein the guide wire is positioned into the ulnar vein Arterial access: From the brachial artery the guide wire is positioned into the ulnar artery

Catheter Overview • • 4 Fr catheter profile Compatible with 5 Fr or 4/5 slender sheath with a. 014” guidewire RX Hydrophilically coated: distal 23 cm Working length from hub to electrode: 40 cm Venous Catheter Arterial Catheter Square Magnetic Regions

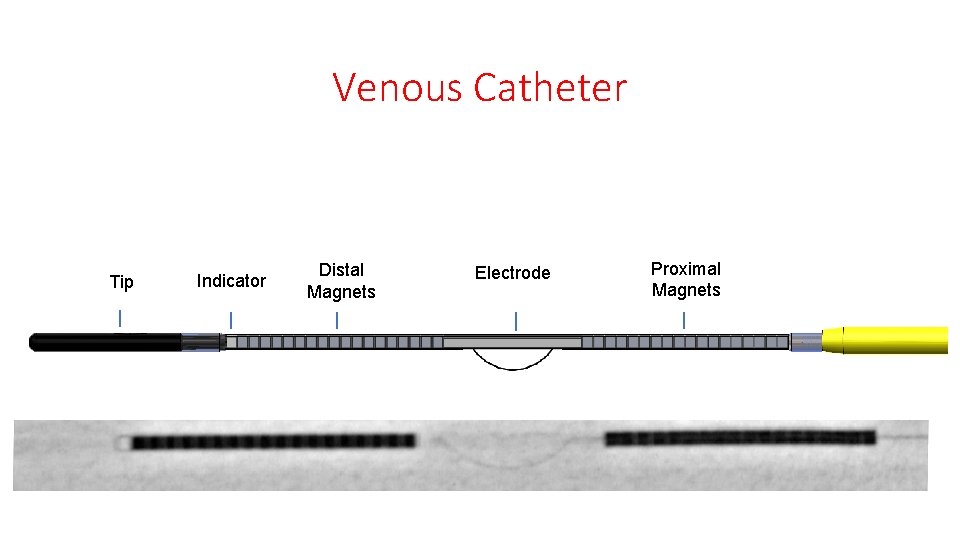
Venous Catheter Tip Indicator Distal Magnets Electrode Proximal Magnets

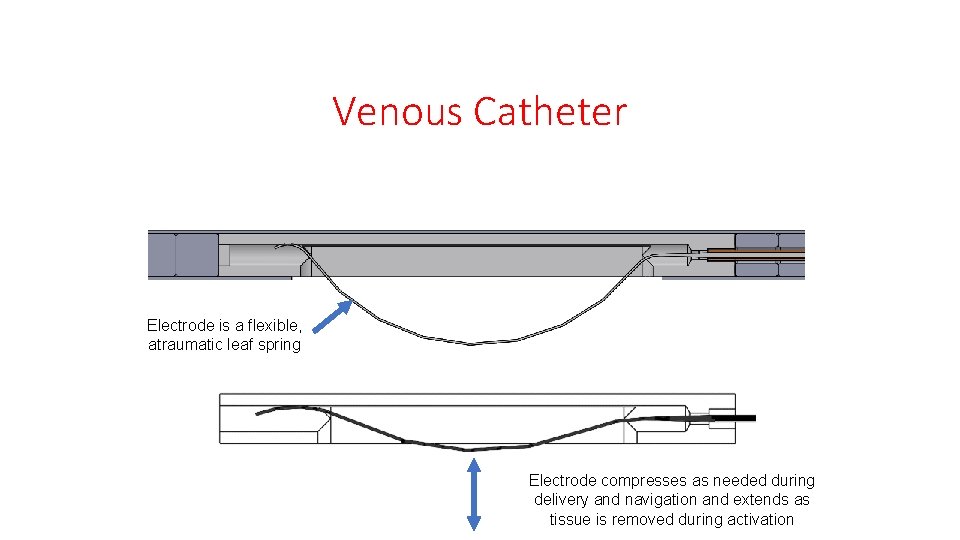
Venous Catheter Electrode is a flexible, atraumatic leaf spring Electrode compresses as needed during delivery and navigation and extends as tissue is removed during activation

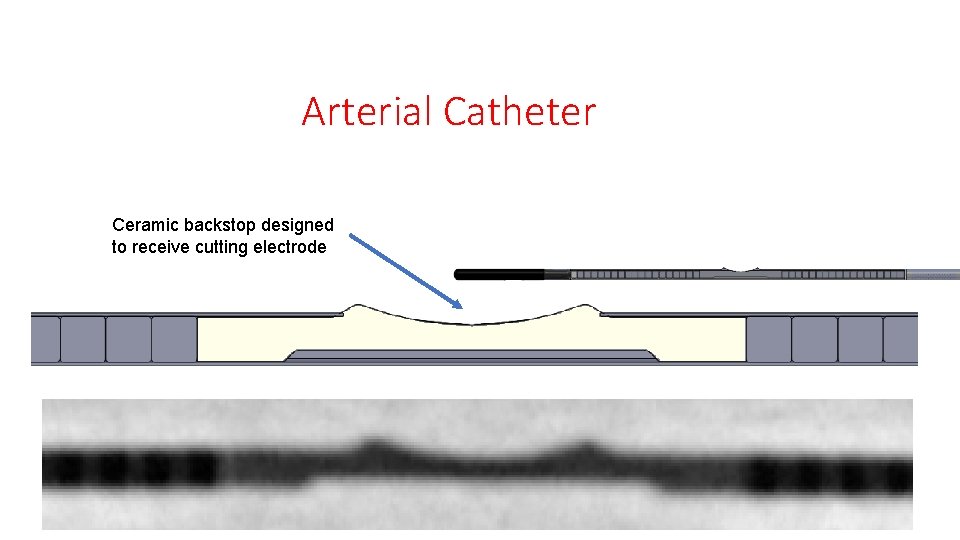
Arterial Catheter Ceramic backstop designed to receive cutting electrode

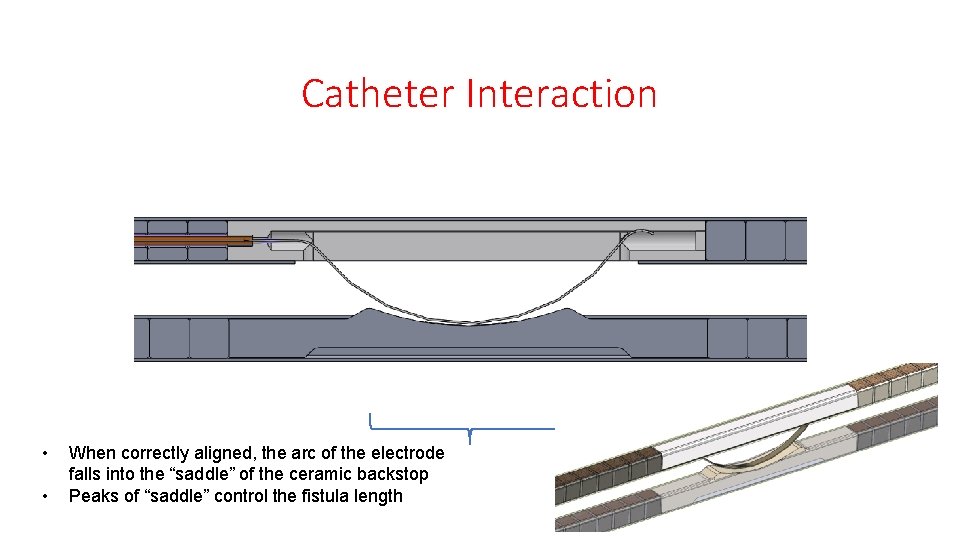
Catheter Interaction • • When correctly aligned, the arc of the electrode falls into the “saddle” of the ceramic backstop Peaks of “saddle” control the fistula length

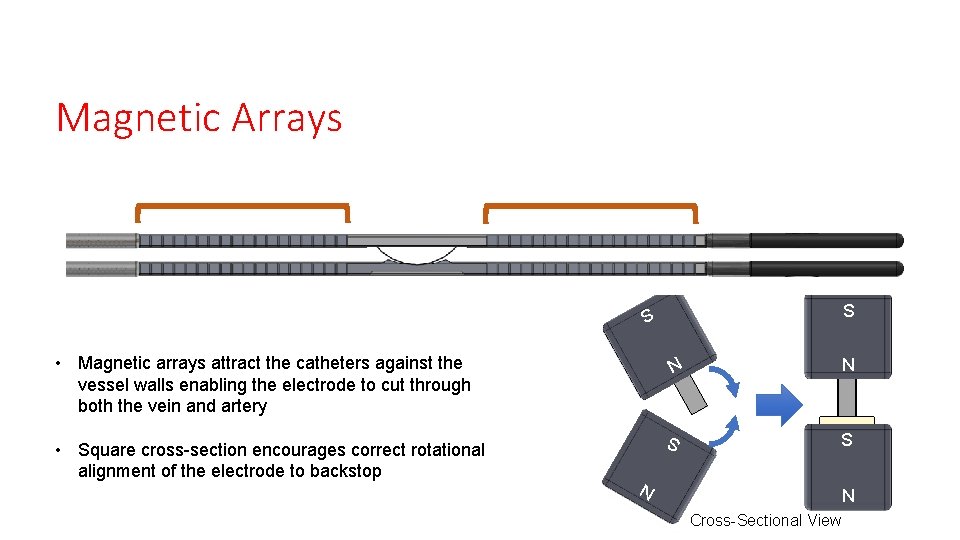
Magnetic Arrays S S N S • Square cross-section encourages correct rotational alignment of the electrode to backstop N • Magnetic arrays attract the catheters against the vessel walls enabling the electrode to cut through both the vein and artery S N N Cross-Sectional View

Magnetic Arrays Caution: Catheters can attract “back-to-back” and “side-to-side” in addition to the correct orientation

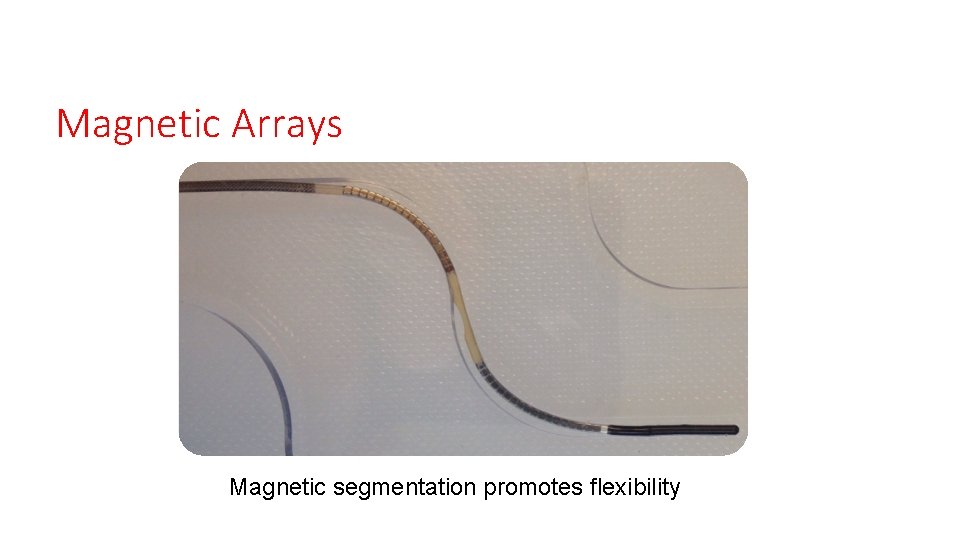
Magnetic Arrays Magnetic segmentation promotes flexibility

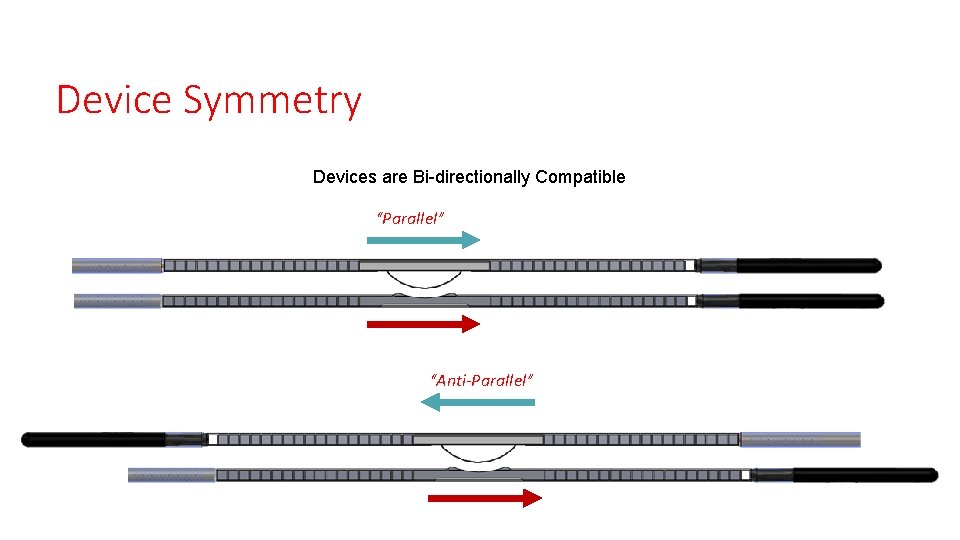
Device Symmetry Devices are Bi-directionally Compatible “Parallel” “Anti-Parallel”

Rotational Indicators

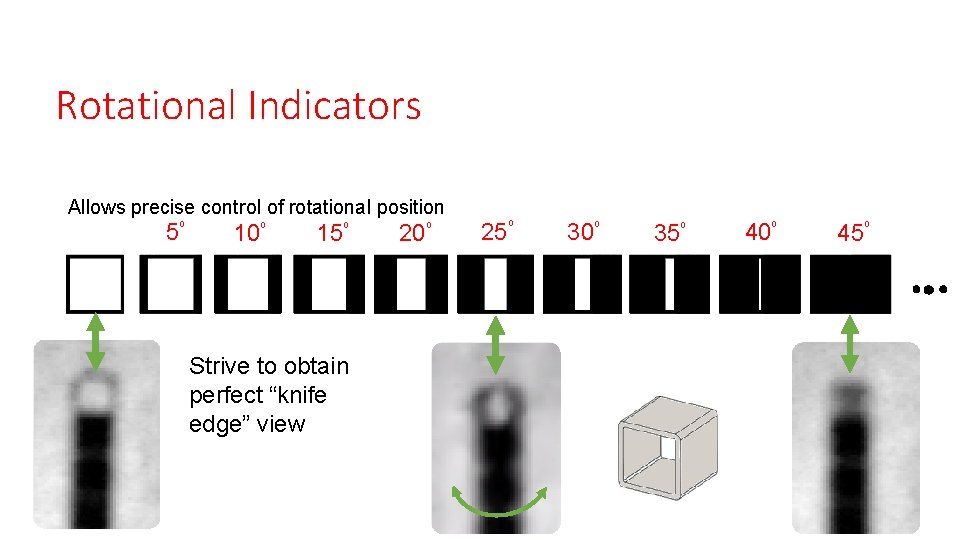
Rotational Indicators Allows precise control of rotational position 5⁰ 10⁰ 15⁰ Strive to obtain perfect “knife edge” view 20⁰ 25⁰ 30⁰ 35⁰ 40⁰ 45⁰


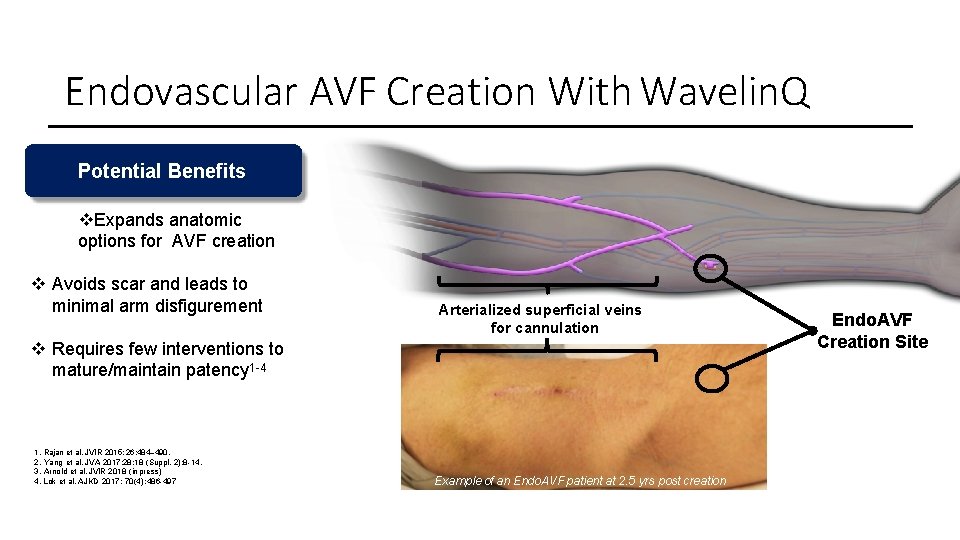
Endovascular AVF Creation With Wavelin. Q Potential Benefits Expands anatomic options for AVF creation Avoids scar and leads to minimal arm disfigurement Arterialized superficial veins for cannulation Requires few interventions to mature/maintain patency 1 -4 1. Rajan et al. JVIR 2015; 26: 484– 490. 2. Yang et al. JVA 2017; 28; 18 (Suppl. 2): 8 -14. 3. Arnold et al. JVIR 2018 (in press) 4. Lok et al. AJKD 2017; 70(4): 486 -497 Example of an Endo. AVF patient at 2. 5 yrs post creation Endo. AVF Creation Site

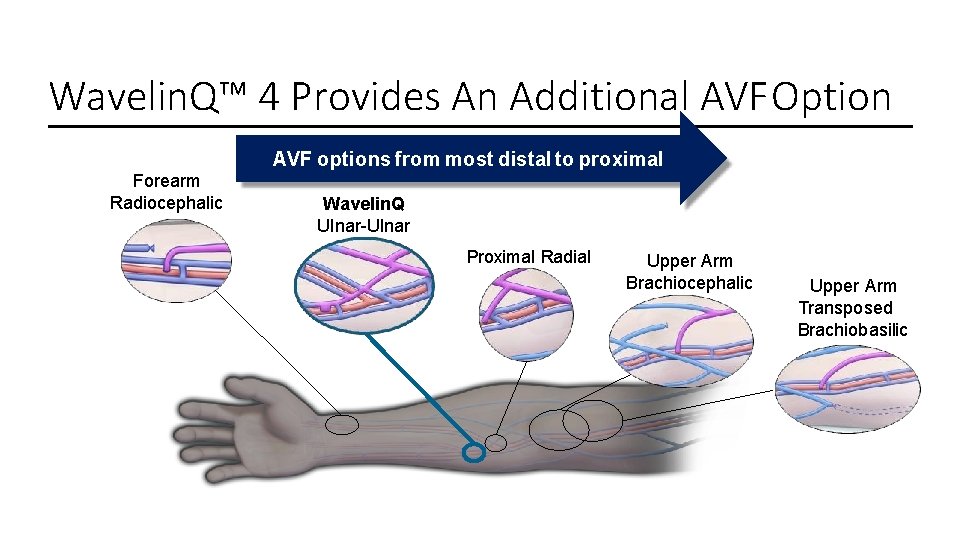
Wavelin. Q™ 4 Provides An Additional AVF Option AVF options from most distal to proximal Forearm Radiocephalic Wavelin. Q Ulnar-Ulnar Proximal Radial Upper Arm Brachiocephalic Upper Arm Transposed Brachiobasilic

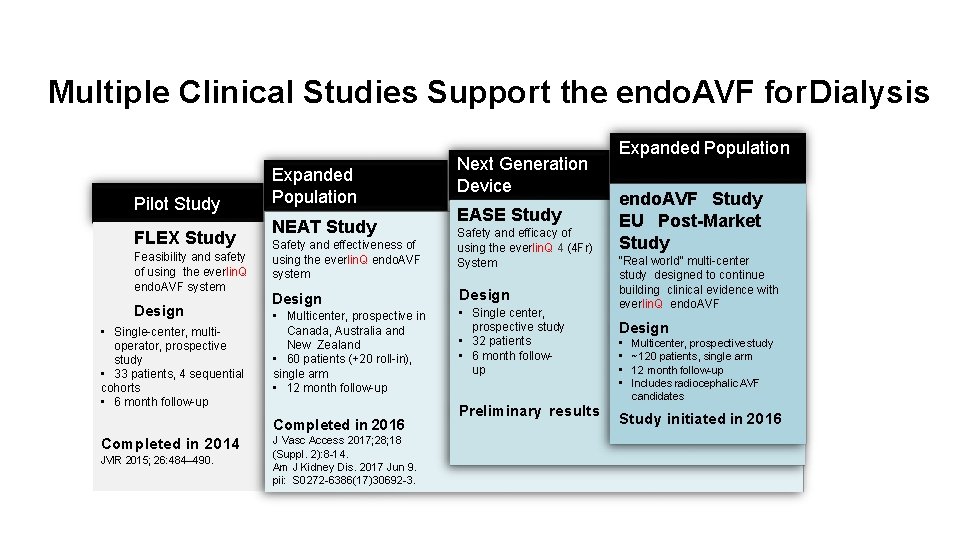
Multiple Clinical Studies Support the endo. AVF for Dialysis Pilot Study FLEX Study Feasibility and safety of using the everlin. Q endo. AVF system Design • Single-center, multioperator, prospective study • 33 patients, 4 sequential cohorts • 6 month follow-up Expanded Population NEAT Study Safety and effectiveness of using the everlin. Q endo. AVF system Design • Multicenter, prospective in Canada, Australia and New Zealand • 60 patients (+20 roll-in), single arm • 12 month follow-up Completed in 2016 Completed in 2014 JVIR 2015; 26: 484– 490. J Vasc Access 2017; 28; 18 (Suppl. 2): 8 -14. Am J Kidney Dis. 2017 Jun 9. pii: S 0272 -6386(17)30692 -3. Next Generation Device EASE Study Safety and efficacy of using the everlin. Q 4 (4 Fr) System Design • Single center, prospective study • 32 patients • 6 month followup Preliminary results Expanded Population endo. AVF Study EU Post-Market Study “Real world” multi-center study designed to continue building clinical evidence with everlin. Q endo. AVF Design • • Multicenter, prospective study ~120 patients, single arm 12 month follow-up Includes radiocephalic AVF candidates Study initiated in 2016

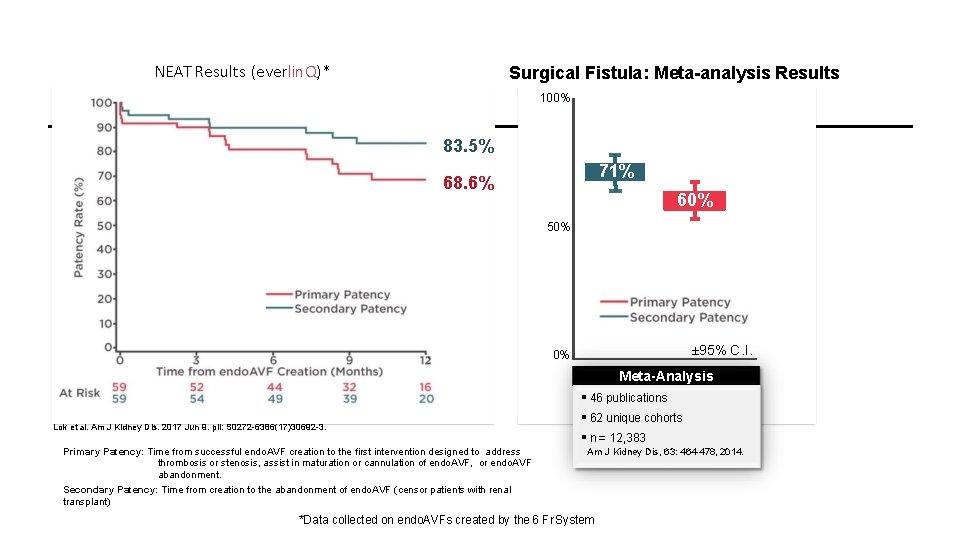
NEAT Results (everlin. Q)* Surgical Fistula: Meta-analysis Results 100% 83. 5% 71% 68. 6% 60% 50% ± 95% C. I. 0% Meta-Analysis Lok et al. Am J Kidney Dis. 2017 Jun 9. pii: S 0272 -6386(17)30692 -3. Primary Patency: Time from successful endo. AVF creation to the first intervention designed to address thrombosis or stenosis, assist in maturation or cannulation of endo. AVF, or endo. AVF abandonment. Secondary Patency: Time from creation to the abandonment of endo. AVF (censor patients with renal transplant) 46 publications 62 unique cohorts n = 12, 383 Am J Kidney Dis, 63: 464 -478, 2014. *Data collected on endo. AVFs created by the 6 Fr System

Few Interventions Required Relative to Surgery Propensity-score matched comparison using a 5% CMS sample of claims data for surgical AVF cohort 4. 0 AVF intervention CVC placement Infection treatment Intervention rate (per patient-year) Procedure Yang S, JVA 2017; 18(Suppl. 2): 8 – 14. 3. 5 3. 0 1. 2 2. 5 0. 4 2. 0 1. 5 1. 0 0. 5 0. 02 0. 1 0. 5 1. 8 endo. AVF Surgical AVF Matched Surgical AVF N=60 endo. AVF N=60 Event rate (per pt-yr) Angioplasty Thrombolysis Thrombectomy Stent placement Embolization/ligation DRIL Thrombin injection Surgical AVF or transposition Revision AVG placement Catheter placement Vascular access related infection (outpatient)* Vascular access related infection (inpatient)* 0. 93 0. 00 0. 20 0. 00 0. 10 0. 00 0. 30 0. 17 0. 07 0. 43 0. 04 0. 02 0. 04 0. 00 0. 13 0. 02 0. 04 0. 11 0. 04 0. 02 0. 11 0. 97 0. 00 0. 27 0. 02 Total Intervention Rate 3. 4 0. 6 * Includes infection due to CVC while AVF maturing

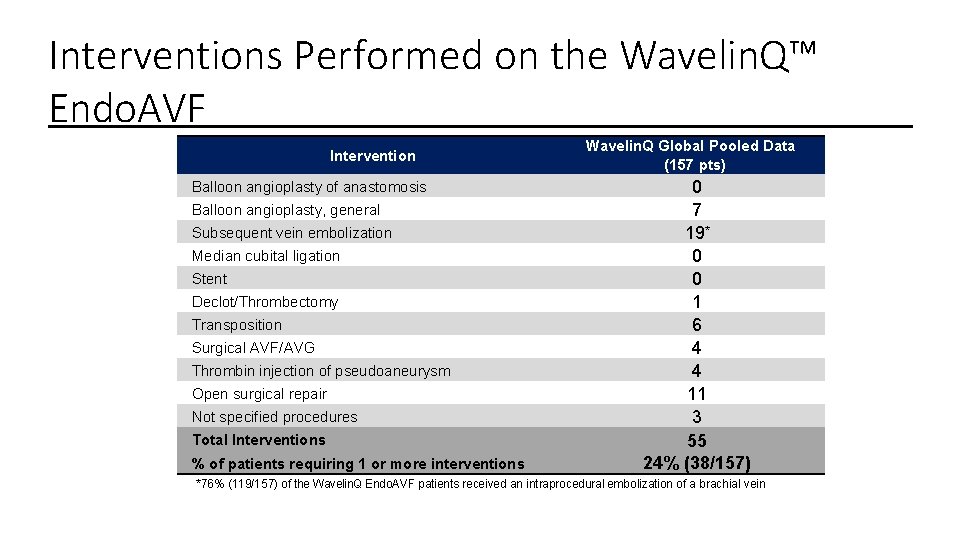
Interventions Performed on the Wavelin. Q™ Endo. AVF Intervention Balloon angioplasty of anastomosis Balloon angioplasty, general Subsequent vein embolization Median cubital ligation Stent Declot/Thrombectomy Transposition Surgical AVF/AVG Thrombin injection of pseudoaneurysm Open surgical repair Not specified procedures Total Interventions % of patients requiring 1 or more interventions Wavelin. Q Global Pooled Data (157 pts) 0 7 19* 0 0 1 6 4 4 11 3 55 24% (38/157) *76% (119/157) of the Wavelin. Q Endo. AVF patients received an intraprocedural embolization of a brachial vein

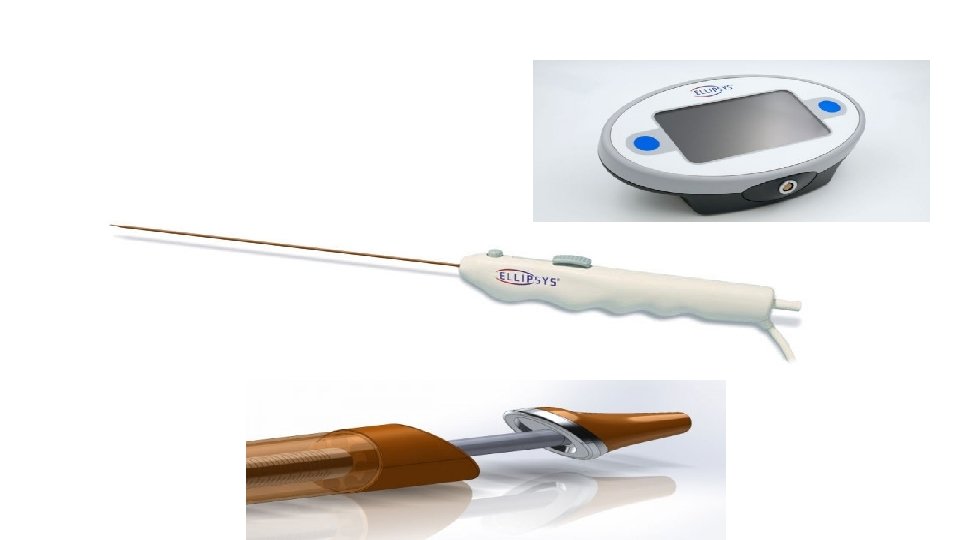
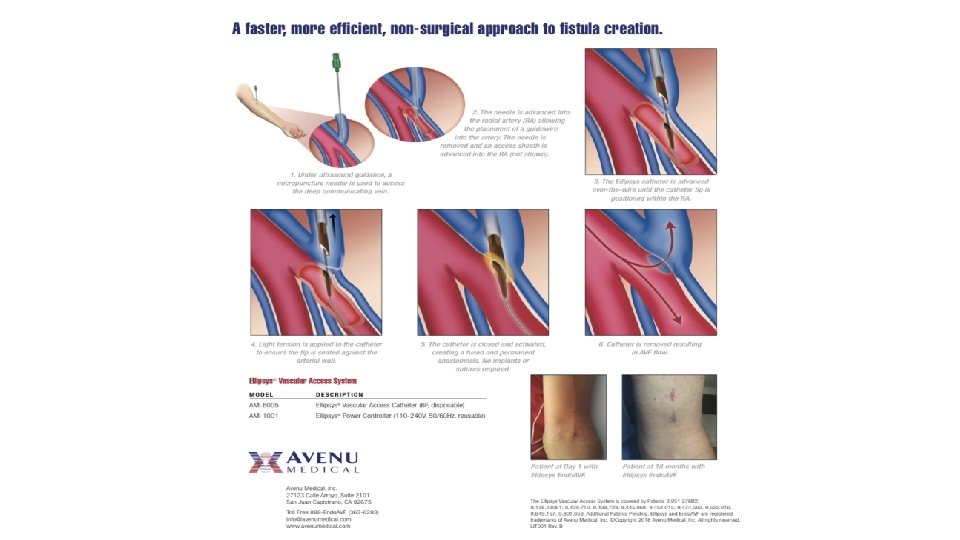
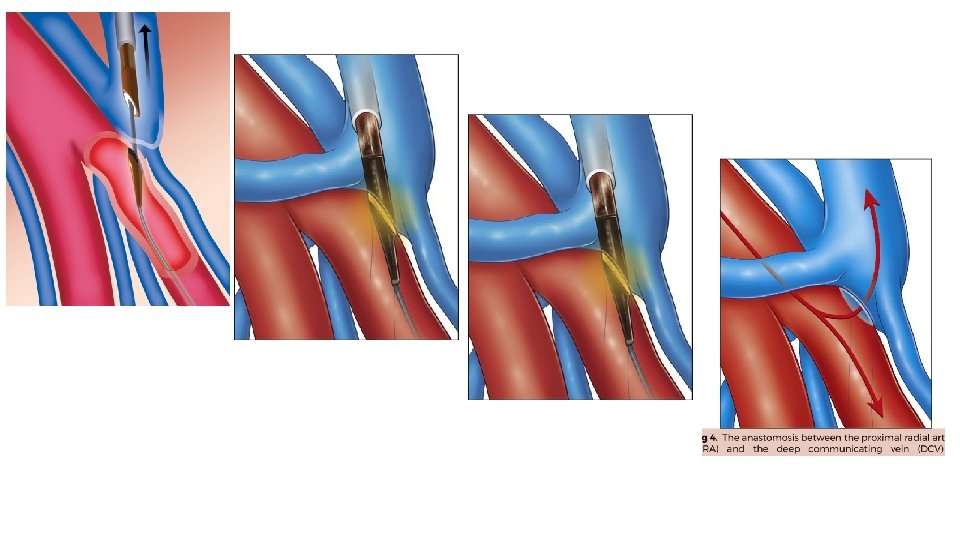
Ellipsys system The Ellipsys endovascular arteriovenous fistula (endo. AVF) system is a single-catheter vascular access system that uses direct heat and pressure to fuse the arterial and venous wall, creating a percutaneous AVF between the proximal radial artery and the deep communicating vein in the proximal forearm





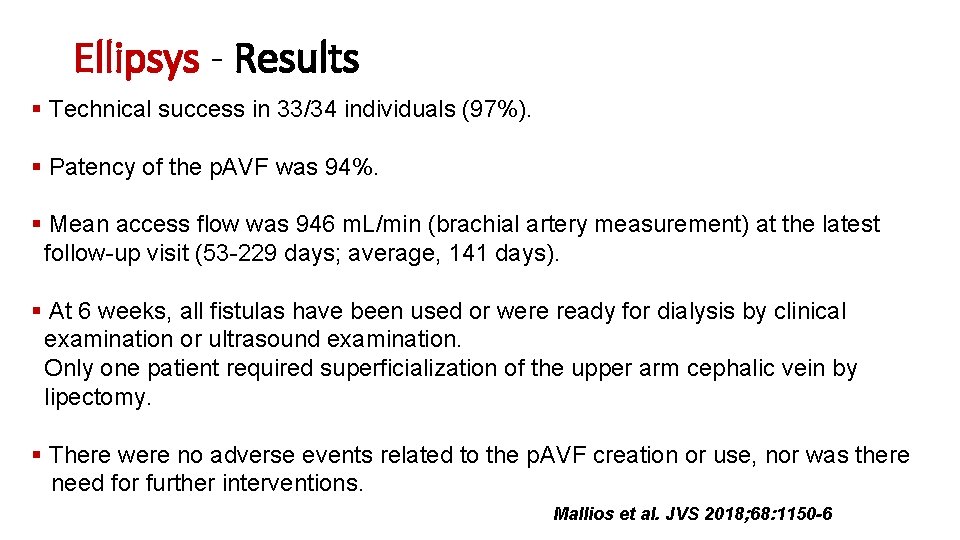
Ellipsys - Results Technical success in 33/34 individuals (97%). Patency of the p. AVF was 94%. Mean access flow was 946 m. L/min (brachial artery measurement) at the latest follow-up visit (53 -229 days; average, 141 days). At 6 weeks, all fistulas have been used or were ready for dialysis by clinical examination or ultrasound examination. Only one patient required superficialization of the upper arm cephalic vein by lipectomy. There were no adverse events related to the p. AVF creation or use, nor was there need for further interventions. Mallios et al. JVS 2018; 68: 1150 -6

SUMMARY • Surgical AVF is the standard of care, but still has some limitations • Endo. AVF creates an AVF without open surgery to minimize vessel trauma – Expands anatomic options for AVF creation – Avoids scar and leads to minimal arm disfigurement – Requires few interventions to mature/maintain patency 1 -4 • Additional benefits to streamlining the overall process of care, which may provide patients with a working AVF sooner 1. Rajan et al. J Vasc Interv Radiol 2015; 26: 484– 490. 2. Yang et al. J Vasc Access 2017; 28; 18 (Suppl. 2): 8 -14. 3. Arnold et al. JVIR 2018 (in press) 4. Lok et al. Am J Kidney Dis. 2017; 70(4): 486 -497

Thanks for the attention

SAVE THE DATE