Creating an Effective Poster Presentation Dr James Mack














- Slides: 18

Creating an Effective Poster Presentation Dr. James Mack Associate Dean of the Graduate School Professor of Chemistry

Oral Communication in Science Written Poster

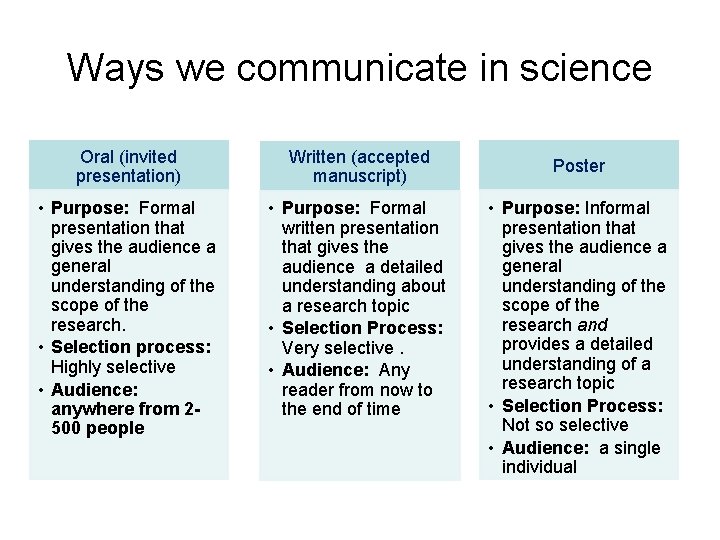
Ways we communicate in science Oral (invited presentation) Written (accepted manuscript) • Purpose: Formal presentation that gives the audience a general understanding of the scope of the research. • Selection process: Highly selective • Audience: anywhere from 2500 people • Purpose: Formal written presentation that gives the audience a detailed understanding about a research topic • Selection Process: Very selective. • Audience: Any reader from now to the end of time Poster • Purpose: Informal presentation that gives the audience a general understanding of the scope of the research and provides a detailed understanding of a research topic • Selection Process: Not so selective • Audience: a single individual

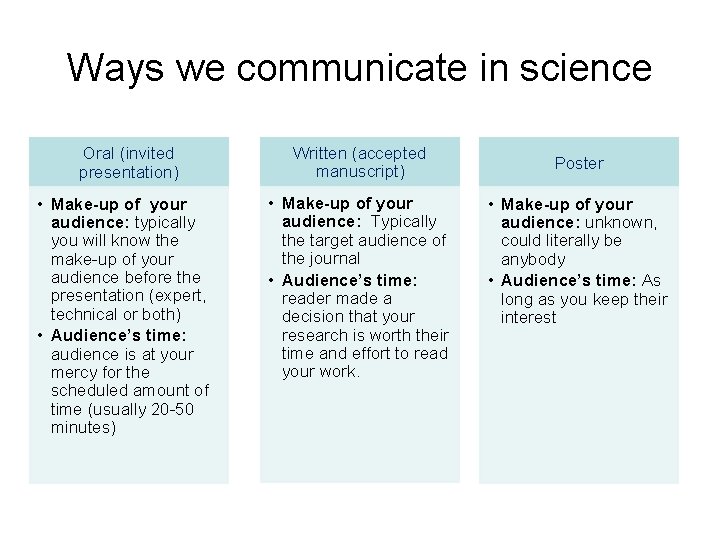
Ways we communicate in science Oral (invited presentation) • Make-up of your audience: typically you will know the make-up of your audience before the presentation (expert, technical or both) • Audience’s time: audience is at your mercy for the scheduled amount of time (usually 20 -50 minutes) Written (accepted manuscript) • Make-up of your audience: Typically the target audience of the journal • Audience’s time: reader made a decision that your research is worth their time and effort to read your work. Poster • Make-up of your audience: unknown, could literally be anybody • Audience’s time: As long as you keep their interest

Venue




Creating an effective poster • Find out the dimensions of the poster board – Make sure you print out a poster that will fit the poster board. – Find out if you will be sharing a board with another person which will allow you to leave space so your poster doesn’t overlap with theirs • Create a title for your poster – This is the first interaction a person will have with your poster. It needs to get their attention but not be too over the top; you don’t want to lose credibility. – Needs to be able to be read from a distance (5 -15 feet away) – Authorship and names of all contributors to the work should be included below along with affiliation, address and contact information for you and corresponding author. Presenter’s name should be underlined. Place an asterisk after the faculty advisor’s name. • Decide on a flow of logic – Begin in a general area that should be understandable to a general audience, starting with an abstract is often a good way to go. – The audience should be able to follow your poster along without you saying a word

Creating an effective poster presentation cont’d • Arrange your poster such that it reflects your flow of logical. – Use of boxes and numbers helps your audience follow your poster – Use as little text as possible, your audience wants to invest the minimum amount of time to gain the maximum amount of return. Pictures do this most effectively. – When you use text (and you will have text on the poster) use a font that can be read from about 5 feet, try to avoid 12 point fonts; 20 - 24 point fonts are typically good to use. • Make your poster visually stimulating, but not overly stimulating – A good use of color can be very attractive but a poor use is a complete turn off. • Don’t forget acknowledgements and references – You want to give credit where it is due

Notes from presentation and exercise: • Ask your college or department for a branded template with the college/dept. /school name. UC templates are also available here: http: //healthnews. uc. edu/branding/? /7258/ • Make sure you change your slide to the actual size of your poster! Design tab > Slide Size > Custom Slide Size • To help with design, turn on Ruler and Gridlines (in the View tab) • Use a clean, simple font, such as Arial • View your poster at 100% to check for grainy pictures, misaligned text/images, etc. • Three on-campus printing options (but check with your department, too, to find out if your dept. or college has a poster printer of its own): • AHC Communication Services (also will print posters on polyester fabric media, which costs a little more per square foot, but the fabric will travel better and it will save you from buying a poster tube) • DAAP Computer Graphics Center • Poster Printer at the CECH Library Media Production Lab

Understanding the energetics of high speed ball mixing with solid-state Diels-Alder reactions by computational methods and by studying ball size and ball material 1. Green Chemistry Green chemistry is the development of processes that reduce or eliminate the use and generation of hazardous substances. In our research we conduct all of our reactions in the solid-state using solvent-free conditions which in turn leads to a reduction in the amount of solvent waste. 5. Calculations 7. Ball material Using density functional theory we were able to calculate the activation barrier. By computational studying the activation energies we will to find out how much energy the mixer/mill approximately generates. If a reaction proceeds, the ball mixer produces at the minimum as much energy as the activation energy. The study about the ball bearings is significant since the amount of force on the compounds can be altered with it, and this is important for the selectivity of reactions. 2. High Speed Ball Mixing Energy in Kcal/mol 2 We conduct all of our solvent-free experiments using the technique of high speed ball milling. In high speed ball milling a ball bearing is sealed in a vial with the starting products and it is shaken at high speeds. The ball bearing acts to break down reagents to particle sizes by which a reactions can occur. Further the ball bearing acts to provide the energy source which governs the reactions. Therefore reactions using this methodology occurs via mechanical energy instead or thermal energy. Reactions are triggered by 0 0. 3 anthracene with maleic anhydride 0 17. 3 yield 9% a mechanical process instead of a thermodynamic process. 3. Research Objective Fig. 1: A SPEX 8000 shaker mill Our research objective is to understand how to control the amount of energy that is generated under our novel conditions. Using the Diels-Alder reaction of Anthracene and Maleic anhydride we sought to determine which variables has the greatest impact on product yield. The three approaches to study the energetics are: - Computational - Ball size and reaction time - Ball material Energy in Kcal/mol 1 6. 9 1 4. 2 0 1 98. methylanthracene 4 with maleic yield: 81% anhydride Reverse reaction yield (35. 3 Kcal/mol): 0% 20. 9, 102 dimethylanthracene with maleic Predicted anhydride The energy generated by the mixer is between 21 and 35 Kcal/mol. 6. Ball size and reaction time %maleic anthracene with yiel Ballanhydride Size diene Fig. 2: Reaction mechanism of studied Diels-Alder reaction 1/4" 9, 10 -dihydroanthracene 9, 10 -α, β-succinic acid anhydride We chose to study the Diels-Alder reactions of anthracene and 9 methylantracene with maleic anhydride because: • The reaction rates of the reactions are only related to thermodynamics • The reactions are not effected by solvents • The reactions do not have by-products Table 3 and 4: Graphs of density and stiffness vs. % yield. Reaction of anthracene with maleic anhydride for 16 hrs. with 1/8” ball. Reactions performed for 16 hrs with 1/8” Fig. 3. Reaction diagrams of Diels-Alder The product of the reaction of 9 -methylanthracene with maleic stainless steel reactions. anhydride was put in the mixer, and it was found that the backball reaction did not take place. Since the forward reactions worked and the reverse reaction did not, we can conclude that: 4. The Reaction No relationship yield >80% With the results from the ball size and reaction time study we would be able see if the amount of energy generated by the mixer can be influence with the size of the ball and time. dieno phile Linear relation, with the exception of aluminum oxide and acrylic Energy Diagrams by B 3 LYP/6 -31 G* Theory d 2. 5 8. 5 6. 4 0. 3 9 -methylanthracene with maleic anhydride 0. 5 hr 16 hrs Ball % % % 1/8" Size yield 1/16" 1/4 71. 6 81. 3 80. 5 no 76. 3 study. 79. 2 77. 6 Table 1 and reaction time Reaction ball 2: Results of ball size 1/8 performed using stainless steel balls. 1/16 82. 2 The results suggest that for the 1/8” ball the force per surface area is the highest, and therefore isno theball most efficient, and that 61. 7 59. 6 increasing the reaction time after 1 hr does not have influence on the yield Yield: no ball < 1/4” < 1/16” < 1/8” Conclusion The amount of energy generated by ball mixing is between 21 and 35 Kcal/mol. The ball size and ball material influence the amount of force on the compound. The 1/8” ball is the most efficient ball of the three sizes. In one hour the ball has collide in all possible directions, and running it for longer than one hour does not increase the yield. In general, the more dense the ball, the greater the force, with Al 2 O 3 and acrylic as exceptions. Future work • Decrease the window of the energy generated by the mixer by studying reaction in with activation energies between 21 and 35 Kcal/mol. • Determine the influence of the volume of the vial on the energy generated by the mixer. Acknowledgments • University of Cincinnati • Dr. James Mack National Science Foundation University of Cincinnati-URC

Utility of Solvent-less High Speed Ball Milling in Free Radical Bromination 1) Background Free Radical Chemistry Green Chemistry is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. In standard solution based synthesis: • Organic soluble free radical initiators are used Examples: benzoyl peroxides and azo compounds • choice of solvent is limited to benzene, which is Carcinogenic or Carbon tetrachloride, which is ozone depleting • Free radical reductions use toxic n-butyl tin hydride • A more benign and environmentally friendly alternative method is needed! Current Project High Speed Vibrational Milling (HSVM) Solid state reactants are placed in a sealed metal vessel with a small bearing in the absence of solvent. The mill is programmed to run for a specific amount of time wherein the ball bearing is oscillated forcing the reactants together. The energy (in the absence of heat) is provided by collisions with the ball. When the reactants come together at the right angle and orientation with respect to the ball, the desired reaction occurs. By using solid state chemistry, we are able to overcome entropy issues as well as make the process greener by eliminating the use of a solvent We look to develop a solvent-free method for conducting radical reactions. Using solvent-free radical chemistry will greatly decrease the use of benzene and carbon tetrachloride in radical chemistry. We are currently investigating the free radical NBS bromination of aromatic compounds on the benzylic position. In these bromination reactions we wish to investigate whether a water soluble free radical initiator such as VAZO 44, can efficiently be used in this process as an alternative to the organic soluble free radical initiators. 2) Results and Discussion 3) Conclusions • Under our conditions, it was found that toluene and 4 -paraiodotoluene underwent bromination on the benzylic position. • We determined that for the two and three ringed systems (1 methyl-napthalene, 9 -methylanthracene and 9, 10 dimethylanthracene), Li. Cl was needed as additive to dilute the system in order to brominate on the benzylic position. The Instrumentation HSVM uses the same principle as the traditional milling and grinding 4) Future Directions of Project Mortar and Pestle Determine the reason why the reaction of 2 methylnapthalene with Li. Cl did not produce Free Radical Bromination. 5) Acknowledgements Spex Certiprep 8000 D Mixer/Mill Canisters and ball-bearing used for the Mixer/Mill University of Cincinnati Department of Chemistry James Mack Group Members University of Cincinnati-URC National Science Foundation

Using high speed ball milling to study the stereochemistry of the Wittig reaction Wittig Reaction Background Isomerization Using HSBM ‘Green’ chemistry strives to use as many environmentally benign methods as possible while doing chemistry E isomer J=12 Hz Total Waste: 11. 5 billion pounds Total Solvent Waste : 3. 7 billion pounds triphenylphosphene oxide • E isomer is thermodynamic product; formed by the slow rotation of the double bond after the triphenylphosphene oxide leaves • Brooks and Scott reported a ratio of 1: 2. 5 E: Z running the Wittig in solution This project focuses on the reduction of solvent waste by using a method called High Speed Ball Milling (HSBM) 1: 27 16. 65 1: 5. 5 33. 30 1: 1. 7 49. 95 1: 1. 5 • MALDI and Electrospray mass spectrometry indicated polymerization as the Experimental Goals: • Find molecules that have easily measurable J-values and chemical shifts • Investigate whether a reaction run in the ball mill gives different isomeric ratios • Polymerization suggests a diradical mechanism pathway • HSBM of the E isomer showed no isomerization, although mass spectrometry indicated polymerization than a reaction run in solution • Observe the effect of HSBM on each isomer Reaction Vial 0. 00 duration of ball milling increased Mechanochemistry SPEX Shaker Mill E: Z Ratio Z isomer J=16 Hz • Z isomer is the kinetic product; formed by the fast leaving of the EPA Industrial Chemical Release Index Time (hr) Future Directions • HSBM of known E isomers to prove isomerization • HSBM of multiple E isomers simultaneously to disprove 2+2 addition • Further understand the isomerization process by putting E isomers in Freezer Mill varying concentrations of sand E: Z 1. 62: 1 In solvent based reactions, heat energy provides the necessary activation energy. Mechanical energy in the form of a ball bearing impacting the sides of a reaction vial provides the energy in mechanochemistry. Reactions are oscillated in a figure-8 motion at 60 Hz in a mill with a ball bearing inside a reaction vial. • Explore time dependence of HSBM on stereoisomeric ratio • Investigate the role triphenylphosphene oxide plays in the reaction References E: Z 1. 40: 1 Concentration Studies 1. 2. 3. http: //www. epa. gov Concas, A. ; Lai, N. ; Pisu, M. ; Cao, G. J. Chem. Eng. Sci. 2006, 61, 3746 -3760 Michele, A. B. ; Lawrence, T. S. J. Am. Chem. Soc. 1999, 121, 5444 -5449 Acknowledgments • NSF-REU Grant CHE 0754114 • University of Cincinnati Department of Chemistry • Dr. James Mack • The Mack Group

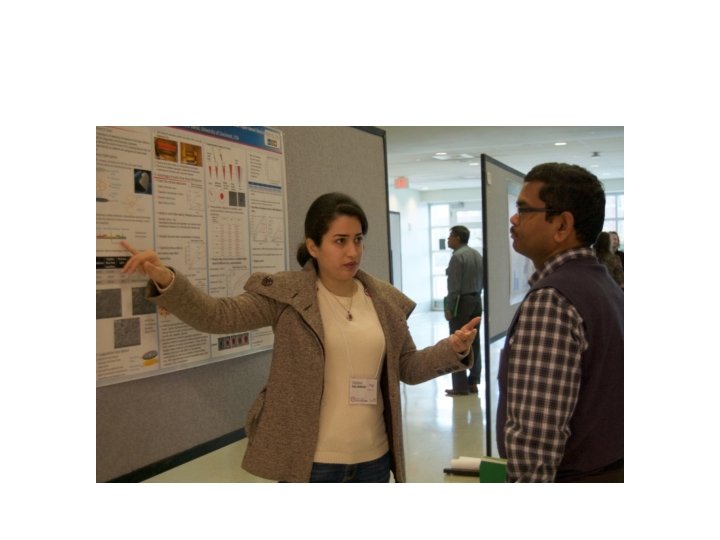
Presenting your poster • Be near your poster – make sure any potential visitor can easily identify you as the poster presenter; wearing a name tag often helps the person with this important task – You can wander but always be in eyesight of your poster • Wait for a person to approach your poster – Poster sessions are meant to be highly informal many poster session has food and drink and people are expected to browse around. – The atmosphere is like that of a yard sale or flea market, there is a lot of people and a lot of posters. Once a person approaches your poster allow them some breathing room, initially – You have no idea what attracted them to your poster, it could be the title, name recognition, or they were waiting for a space to clear up at a poster near yours.

Presenting your poster cont’d • Stick to the flow of logic outlined in your poster design – Your flow of logical and your presentation should be in sync • initiating a conversation – “Hello my name is Thomas Smith, would you like me to walk you through my poster? ” – Try to get information about the visitor so you can determine whether you should be more general or more specific with your presentation • “Be quick but don’t hurry” – the visitor wants time to visit other posters so you need to be succinct but you don’t want to rush through your presentation just to get through it.

Other things to note • • • Try to stick with one person at a time – it is often for another visitor to come to your poster midway through your presentation to someone else. Acknowledge they exist but don’t restart the presentation. If they have questions they will wait until you are done with the first person Be on time – Somebody may specifically be targeting your poster because they want more details or want to start a collaboration. Wear something comfortable but not too comfortable – Poster sessions can last two hours or more and you will be standing the whole time You are also a visitor – Feel free at lulls to walk and view other people’s poster. You should take advantage of the research that is available to you. Try to get ideas – Visitors may have a different view of your research that you have not thought about previously. Be receptive to advice and have a pen handy to exchange information.

Other resources • Do’s and don’ts of poster presentations • Ten Simple Rules for a Good Poster Presentation • Designing effective posters • Other internet sites • UC Branding • ACS surviving your first poster session