Creactive Protein Rise is Associated with the Development
- Slides: 2

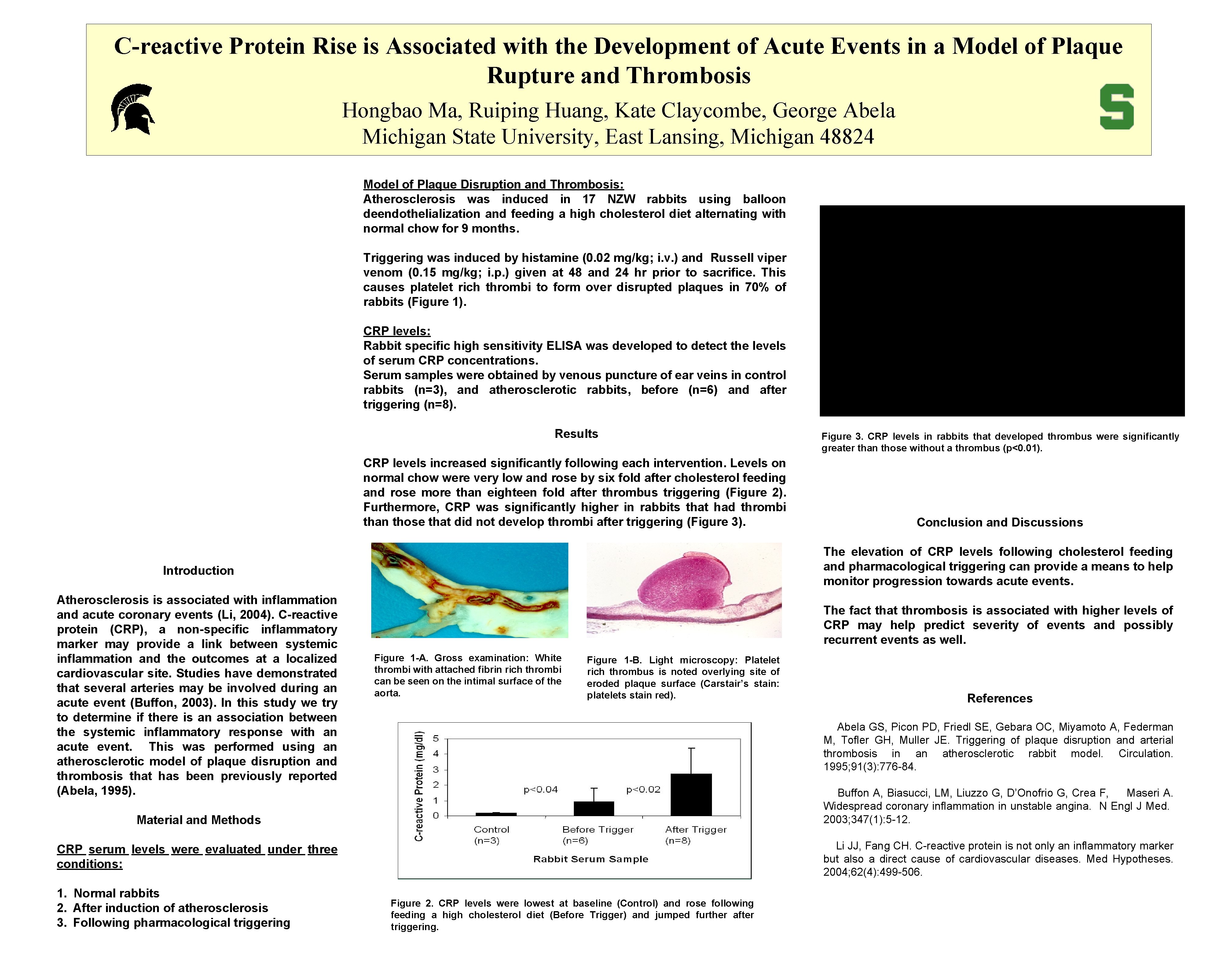
C-reactive Protein Rise is Associated with the Development of Acute Events in a Model of Plaque Rupture and Thrombosis Hongbao Ma, Ruiping Huang, Kate Claycombe, George Abela Michigan State University, East Lansing, Michigan 48824 Model of Plaque Disruption and Thrombosis: Atherosclerosis was induced in 17 NZW rabbits using balloon deendothelialization and feeding a high cholesterol diet alternating with normal chow for 9 months. Triggering was induced by histamine (0. 02 mg/kg; i. v. ) and Russell viper venom (0. 15 mg/kg; i. p. ) given at 48 and 24 hr prior to sacrifice. This causes platelet rich thrombi to form over disrupted plaques in 70% of rabbits (Figure 1). CRP levels: Rabbit specific high sensitivity ELISA was developed to detect the levels of serum CRP concentrations. Serum samples were obtained by venous puncture of ear veins in control rabbits (n=3), and atherosclerotic rabbits, before (n=6) and after triggering (n=8). Results CRP levels increased significantly following each intervention. Levels on normal chow were very low and rose by six fold after cholesterol feeding and rose more than eighteen fold after thrombus triggering (Figure 2). Furthermore, CRP was significantly higher in rabbits that had thrombi than those that did not develop thrombi after triggering (Figure 3). The fact that thrombosis is associated with higher levels of CRP may help predict severity of events and possibly recurrent events as well. Figure 1 -A. Gross examination: White thrombi with attached fibrin rich thrombi can be seen on the intimal surface of the aorta. Figure 1 -B. Light microscopy: Platelet rich thrombus is noted overlying site of eroded plaque surface (Carstair’s stain: platelets stain red). References Abela GS, Picon PD, Friedl SE, Gebara OC, Miyamoto A, Federman M, Tofler GH, Muller JE. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation. 1995; 91(3): 776 -84. Buffon A, Biasucci, LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2003; 347(1): 5 -12. Material and Methods Li JJ, Fang CH. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses. 2004; 62(4): 499 -506. CRP serum levels were evaluated under three conditions: 1. Normal rabbits 2. After induction of atherosclerosis 3. Following pharmacological triggering Conclusion and Discussions The elevation of CRP levels following cholesterol feeding and pharmacological triggering can provide a means to help monitor progression towards acute events. Introduction Atherosclerosis is associated with inflammation and acute coronary events (Li, 2004). C-reactive protein (CRP), a non-specific inflammatory marker may provide a link between systemic inflammation and the outcomes at a localized cardiovascular site. Studies have demonstrated that several arteries may be involved during an acute event (Buffon, 2003). In this study we try to determine if there is an association between the systemic inflammatory response with an acute event. This was performed using an atherosclerotic model of plaque disruption and thrombosis that has been previously reported (Abela, 1995). Figure 3. CRP levels in rabbits that developed thrombus were significantly greater than those without a thrombus (p<0. 01). Figure 2. CRP levels were lowest at baseline (Control) and rose following feeding a high cholesterol diet (Before Trigger) and jumped further after triggering.

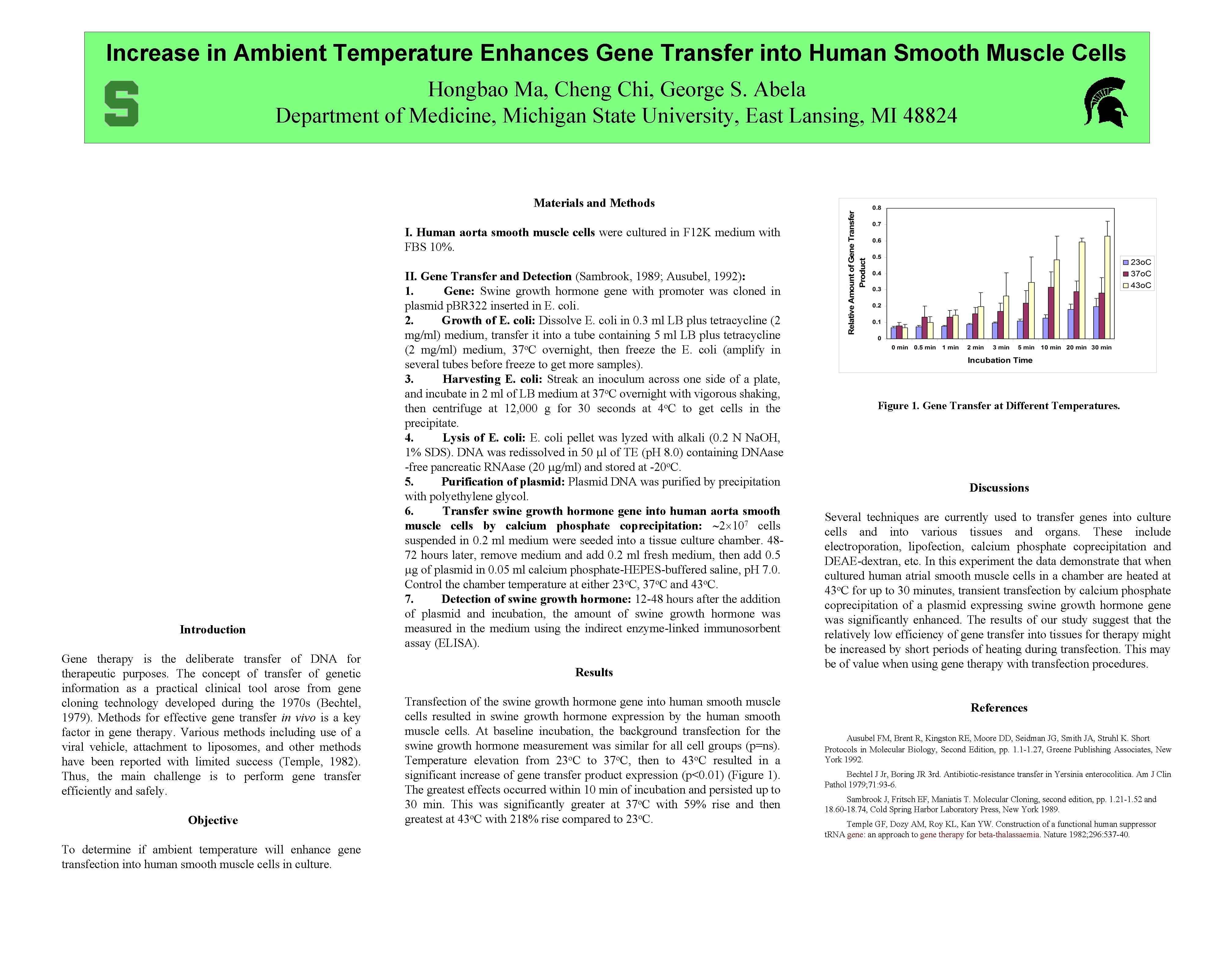
Increase in Ambient Temperature Enhances Gene Transfer into Human Smooth Muscle Cells Hongbao Ma, Cheng Chi, George S. Abela Department of Medicine, Michigan State University, East Lansing, MI 48824 Materials and Methods I. Human aorta smooth muscle cells were cultured in F 12 K medium with FBS 10%. Introduction Gene therapy is the deliberate transfer of DNA for therapeutic purposes. The concept of transfer of genetic information as a practical clinical tool arose from gene cloning technology developed during the 1970 s (Bechtel, 1979). Methods for effective gene transfer in vivo is a key factor in gene therapy. Various methods including use of a viral vehicle, attachment to liposomes, and other methods have been reported with limited success (Temple, 1982). Thus, the main challenge is to perform gene transfer efficiently and safely. Objective To determine if ambient temperature will enhance gene transfection into human smooth muscle cells in culture. II. Gene Transfer and Detection (Sambrook, 1989; Ausubel, 1992): 1. Gene: Swine growth hormone gene with promoter was cloned in plasmid p. BR 322 inserted in E. coli. 2. Growth of E. coli: Dissolve E. coli in 0. 3 ml LB plus tetracycline (2 mg/ml) medium, transfer it into a tube containing 5 ml LB plus tetracycline (2 mg/ml) medium, 37 o. C overnight, then freeze the E. coli (amplify in several tubes before freeze to get more samples). 3. Harvesting E. coli: Streak an inoculum across one side of a plate, and incubate in 2 ml of LB medium at 37 o. C overnight with vigorous shaking, then centrifuge at 12, 000 g for 30 seconds at 4 o. C to get cells in the precipitate. 4. Lysis of E. coli: E. coli pellet was lyzed with alkali (0. 2 N Na. OH, 1% SDS). DNA was redissolved in 50 l of TE (p. H 8. 0) containing DNAase -free pancreatic RNAase (20 g/ml) and stored at -20 o. C. 5. Purification of plasmid: Plasmid DNA was purified by precipitation with polyethylene glycol. 6. Transfer swine growth hormone gene into human aorta smooth muscle cells by calcium phosphate coprecipitation: 2 107 cells suspended in 0. 2 ml medium were seeded into a tissue culture chamber. 4872 hours later, remove medium and add 0. 2 ml fresh medium, then add 0. 5 g of plasmid in 0. 05 ml calcium phosphate-HEPES-buffered saline, p. H 7. 0. Control the chamber temperature at either 23 o. C, 37 o. C and 43 o. C. 7. Detection of swine growth hormone: 12 -48 hours after the addition of plasmid and incubation, the amount of swine growth hormone was measured in the medium using the indirect enzyme-linked immunosorbent assay (ELISA). Results Transfection of the swine growth hormone gene into human smooth muscle cells resulted in swine growth hormone expression by the human smooth muscle cells. At baseline incubation, the background transfection for the swine growth hormone measurement was similar for all cell groups (p=ns). Temperature elevation from 23 o. C to 37 o. C, then to 43 o. C resulted in a significant increase of gene transfer product expression (p<0. 01) (Figure 1). The greatest effects occurred within 10 min of incubation and persisted up to 30 min. This was significantly greater at 37 o. C with 59% rise and then greatest at 43 o. C with 218% rise compared to 23 o. C. Figure 1. Gene Transfer at Different Temperatures. Discussions Several techniques are currently used to transfer genes into culture cells and into various tissues and organs. These include electroporation, lipofection, calcium phosphate coprecipitation and DEAE-dextran, etc. In this experiment the data demonstrate that when cultured human atrial smooth muscle cells in a chamber are heated at 43 o. C for up to 30 minutes, transient transfection by calcium phosphate coprecipitation of a plasmid expressing swine growth hormone gene was significantly enhanced. The results of our study suggest that the relatively low efficiency of gene transfer into tissues for therapy might be increased by short periods of heating during transfection. This may be of value when using gene therapy with transfection procedures. References Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology, Second Edition, pp. 1. 1 -1. 27, Greene Publishing Associates, New York 1992. Bechtel J Jr, Boring JR 3 rd. Antibiotic-resistance transfer in Yersinia enterocolitica. Am J Clin Pathol 1979; 71: 93 -6. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, second edition, pp. 1. 21 -1. 52 and 18. 60 -18. 74, Cold Spring Harbor Laboratory Press, New York 1989. Temple GF, Dozy AM, Roy KL, Kan YW. Construction of a functional human suppressor t. RNA gene: an approach to gene therapy for beta-thalassaemia. Nature 1982; 296: 537 -40.