CRC treatment guidelines Guidelines for the treatment of




- Slides: 10

CRC: treatment guidelines Guidelines for the treatment of advanced colon and rectal cancers 1. NCCN Clinical Practice Guideline in Oncology. Rectal Cancer, Version 1. 2020; 2. NCCN Clinical Practice Guideline in Oncology. Colon Cancer, Version 1. 2020; 3. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

Introduction • This slide deck summarises the recommended treatment pathways provided by ESMO and NCCN for locally advanced or metastatic CRC, which includes rectal and colon cancers 1– 3 • Please refer to the full guidelines for more information on: – Diagnostic work-up and follow-up – Treatment of early- and intermediate-stage disease – Details of specific treatment regimens CRC, colorectal cancer; ESMO, European Society for Medical Oncology; NCCN, National Comprehensive Cancer Network. 1. NCCN Clinical Practice Guideline in Oncology. Rectal Cancer, Version 1. 2020; 2. NCCN Clinical Practice Guideline in Oncology. Colon Cancer, Version 1. 2020; 3. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

ESMO consensus guidelines Summary of management guidelines for patients with metastatic colorectal cancer 1. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

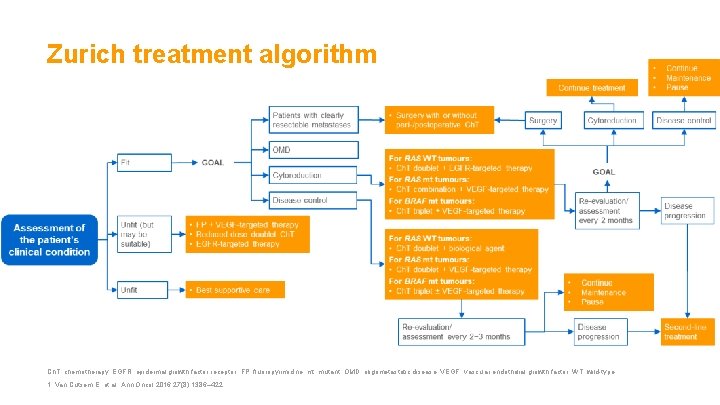
Zurich treatment algorithm Ch. T, chemotherapy; EGFR, epidermal growth factor receptor; FP, fluoropyrimidine; mt, mutant; OMD, oligometastatic disease; VEGF, vascular endothelial growth factor; WT, wild-type. 1. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

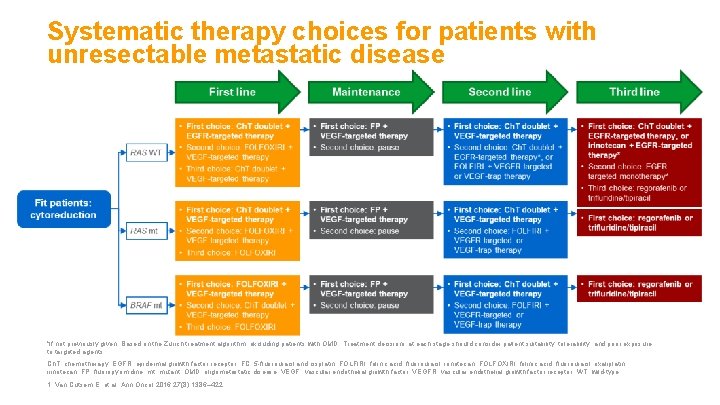
Systematic therapy choices for patients with unresectable metastatic disease *If not previously given. Based on the Zurich treatment algorithm; excluding patients with OMD. Treatment decisions at each stage should consider patient suitability, tolerability, and prior exposure to targeted agents. Ch. T, chemotherapy; EGFR, epidermal growth factor receptor; FC, 5 -fluorouracil and cisplatin; FOLFIRI, folinic acid, fluorouracil, irinotecan; FOLFOXIRI, folinic acid, fluorouracil, oxaliplatin, irinotecan; FP, fluoropyrimidine; mt, mutant; OMD, oligometastatic disease; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; WT, wild-type. 1. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

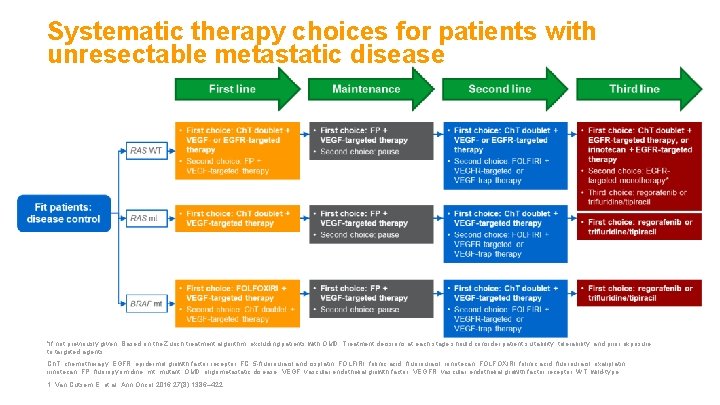
Systematic therapy choices for patients with unresectable metastatic disease *If not previously given. Based on the Zurich treatment algorithm; excluding patients with OMD. Treatment decisions at each stage should consider patient suitability, tolerability, and prior exposure to targeted agents. Ch. T, chemotherapy; EGFR, epidermal growth factor receptor; FC, 5 -fluorouracil and cisplatin; FOLFIRI, folinic acid, fluorouracil, irinotecan; FOLFOXIRI, folinic acid, fluorouracil, oxaliplatin, irinotecan; FP, fluoropyrimidine; mt, mutant; OMD, oligometastatic disease; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; WT, wild-type. 1. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.

NCCN Clinical Practice Guidelines Treatment considerations for advanced colon and rectal cancers 1. NCCN Clinical Practice Guideline in Oncology. Rectal Cancer, Version 1. 2020; 2. NCCN Clinical Practice Guideline in Oncology. Colon Cancer, Version 1. 2020.

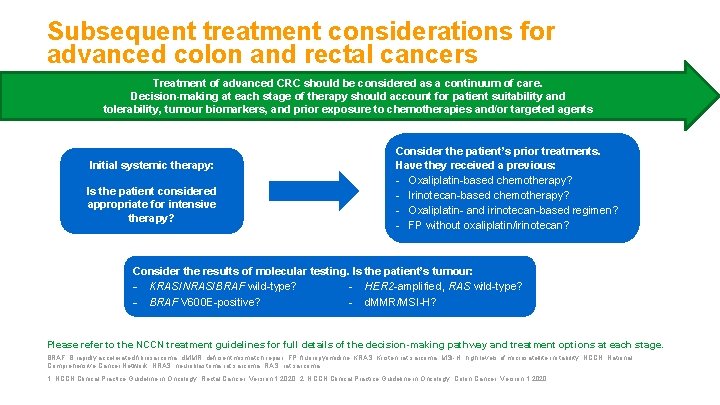
Subsequent treatment considerations for advanced colon and rectal cancers Treatment of advanced CRC should be considered as a continuum of care. Decision-making at each stage of therapy should account for patient suitability and tolerability, tumour biomarkers, and prior exposure to chemotherapies and/or targeted agents Initial systemic therapy: Is the patient considered appropriate for intensive therapy? Consider the patient’s prior treatments. Have they received a previous: - Oxaliplatin-based chemotherapy? - Irinotecan-based chemotherapy? - Oxaliplatin- and irinotecan-based regimen? - FP without oxaliplatin/irinotecan? Consider the results of molecular testing. Is the patient’s tumour: - KRAS/NRAS/BRAF wild-type? - HER 2 -amplified, RAS wild-type? - BRAF V 600 E-positive? - d. MMR/MSI-H? Please refer to the NCCN treatment guidelines for full details of the decision-making pathway and treatment options at each stage. BRAF, B rapidly accelerated fibrosarcoma; d. MMR, deficient mismatch repair; FP, fluoropyrimidine; KRAS, Kirsten rat sarcoma; MSI-H, high levels of microsatellite instability; NCCN, National Comprehensive Cancer Network; NRAS, neuroblastoma rat sarcoma; RAS, rat sarcoma. 1. NCCN Clinical Practice Guideline in Oncology. Rectal Cancer, Version 1. 2020; 2. NCCN Clinical Practice Guideline in Oncology. Colon Cancer, Version 1. 2020.

Summary

Summary • ESMO and NCCN guidelines aim to provide guidelines for the diagnosis, treatment and follow-up of CRC based on the findings of evidence-based medicine • Both sets of guidelines provide specific treatment recommendations for patients with defined molecular markers (including RAS, BRAF, MSI and HER 2) • Management of CRC can be considered a ‘continuum of care’, and treatment decisions at each stage should take into account the patient’s clinical status and treatment goals CRC, colorectal cancer; ESMO, European Society for Medical Oncology; HER 2, human epidermal growth factor receptor 2; MSI, microsatellite instability; NCCN, National Comprehensive Cancer Network. 1. Van Cutsem E, et al. Ann Oncol 2016; 27(8): 1386– 422.